What Temp Does Salt Melt Ice Celsius . pure water freezes at 32°f (0°c). But how does salt do it? More water is melting than freezing at the normal freezing temperature, and so the ice. at what temperature does salt melt ice? more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. First, it’s important to understand a bit about h 2 o in the winter. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. that upsets the balance; Water with salt (or any other substance in it) will freeze at some lower temperature. when salt is applied to ice, it disrupts the freezing process.
from chem.libretexts.org
when salt is applied to ice, it disrupts the freezing process. more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. pure water freezes at 32°f (0°c). But how does salt do it? that upsets the balance; More water is melting than freezing at the normal freezing temperature, and so the ice. This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. at what temperature does salt melt ice?
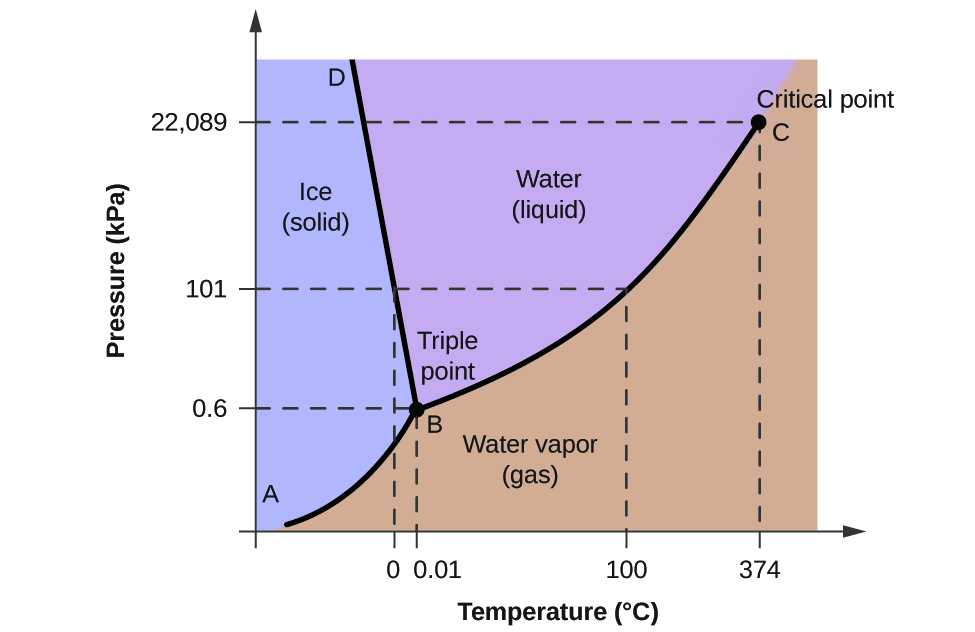
10.4 Phase Diagrams Chemistry LibreTexts
What Temp Does Salt Melt Ice Celsius First, it’s important to understand a bit about h 2 o in the winter. when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. at what temperature does salt melt ice? Water with salt (or any other substance in it) will freeze at some lower temperature. But how does salt do it? that upsets the balance; when salt is applied to ice, it disrupts the freezing process. This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. pure water freezes at 32°f (0°c). More water is melting than freezing at the normal freezing temperature, and so the ice. First, it’s important to understand a bit about h 2 o in the winter. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice.
From tinhte.vn
Băng trôi tan làm nước biển dâng lên, khác đá tan trong ly. 27 năm qua What Temp Does Salt Melt Ice Celsius First, it’s important to understand a bit about h 2 o in the winter. More water is melting than freezing at the normal freezing temperature, and so the ice. more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. This happens because salt lowers the freezing point of water,. What Temp Does Salt Melt Ice Celsius.
From dxovuuibg.blob.core.windows.net
How Does Salt Melt Snow And Ice at William Carbajal blog What Temp Does Salt Melt Ice Celsius Water with salt (or any other substance in it) will freeze at some lower temperature. More water is melting than freezing at the normal freezing temperature, and so the ice. First, it’s important to understand a bit about h 2 o in the winter. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46. What Temp Does Salt Melt Ice Celsius.
From www.thoughtco.com
Why Does Salt Melt Ice? Understanding How It Works What Temp Does Salt Melt Ice Celsius This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. Water with salt (or any other substance in it) will freeze at some lower temperature. But how does salt do it? pure water freezes at 32°f (0°c). More. What Temp Does Salt Melt Ice Celsius.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas What Temp Does Salt Melt Ice Celsius Water with salt (or any other substance in it) will freeze at some lower temperature. More water is melting than freezing at the normal freezing temperature, and so the ice. But how does salt do it? when salt is applied to ice, it disrupts the freezing process. at what temperature does salt melt ice? when salt is. What Temp Does Salt Melt Ice Celsius.
From www.science-sparks.com
Why does salt melt ice? What Temp Does Salt Melt Ice Celsius when salt is applied to ice, it disrupts the freezing process. More water is melting than freezing at the normal freezing temperature, and so the ice. that upsets the balance; Water with salt (or any other substance in it) will freeze at some lower temperature. pure water freezes at 32°f (0°c). But how does salt do it?. What Temp Does Salt Melt Ice Celsius.
From www.metoffice.gov.uk
Sea ice an overview Met Office What Temp Does Salt Melt Ice Celsius This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. that upsets the balance; at what temperature does. What Temp Does Salt Melt Ice Celsius.
From exokjpmgr.blob.core.windows.net
Will Salt Melt Ice at Richard Weaver blog What Temp Does Salt Melt Ice Celsius More water is melting than freezing at the normal freezing temperature, and so the ice. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. at what temperature does salt melt ice? when salt is added to ice, it dissolves into the water present on the ice's surface, creating. What Temp Does Salt Melt Ice Celsius.
From snowicesalt.com
Ice Melt Comparison Chart Snow & Ice Salt & Chemicals Unlimited, LLC What Temp Does Salt Melt Ice Celsius when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. pure water freezes at 32°f (0°c). Water with salt (or any other substance in it). What Temp Does Salt Melt Ice Celsius.
From studylib.net
How Does Salt Melt ice and make Ice Cream What Temp Does Salt Melt Ice Celsius more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. But how does salt do it? At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. pure water freezes at 32°f (0°c). More water is melting than freezing at. What Temp Does Salt Melt Ice Celsius.
From dxotswbpt.blob.core.windows.net
Will Salt Water Melt Ice at Jimmy Chang blog What Temp Does Salt Melt Ice Celsius This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. at what temperature does salt melt ice? pure water freezes at 32°f (0°c). Water with salt (or any other substance in it) will freeze at some lower. What Temp Does Salt Melt Ice Celsius.
From ar.inspiredpencil.com
Melting Ice With Salt What Temp Does Salt Melt Ice Celsius Water with salt (or any other substance in it) will freeze at some lower temperature. First, it’s important to understand a bit about h 2 o in the winter. pure water freezes at 32°f (0°c). This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees. What Temp Does Salt Melt Ice Celsius.
From safepaw.com
What Temperature Does Salt Melt Ice? Science Behind Salt and Ice What Temp Does Salt Melt Ice Celsius At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. that upsets the balance; pure water freezes at 32°f (0°c). First, it’s important to understand a bit about h 2 o in the winter. More water is melting than freezing at the normal freezing temperature, and so the ice.. What Temp Does Salt Melt Ice Celsius.
From littlebinsforlittlehands.com
What Makes Ice Melt Faster? Little Bins for Little Hands What Temp Does Salt Melt Ice Celsius more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. More water is melting than freezing at the normal freezing temperature, and so the ice. that upsets the balance;. What Temp Does Salt Melt Ice Celsius.
From exoanuokq.blob.core.windows.net
Ice Salt To Melt Snow at Eric Arnold blog What Temp Does Salt Melt Ice Celsius at what temperature does salt melt ice? First, it’s important to understand a bit about h 2 o in the winter. But how does salt do it? when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. more than 20 million tons of salt are. What Temp Does Salt Melt Ice Celsius.
From huntingwaterfalls.com
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED What Temp Does Salt Melt Ice Celsius More water is melting than freezing at the normal freezing temperature, and so the ice. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. But how does salt do it? Water with salt (or any other substance in it) will freeze at some lower temperature. more than 20 million. What Temp Does Salt Melt Ice Celsius.
From magicgouveiapetters.z21.web.core.windows.net
Why Does Adding Salt To Ice Make It Melt What Temp Does Salt Melt Ice Celsius more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. pure water freezes at 32°f (0°c). when salt is applied to ice, it disrupts the freezing process. But how does salt do it? when salt is added to ice, it dissolves into the water present on. What Temp Does Salt Melt Ice Celsius.
From www.numerade.com
A portion of the H2ONaCl phase diagram is shown. Using the diagram What Temp Does Salt Melt Ice Celsius when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. pure water freezes at 32°f (0°c). when salt is applied to ice, it disrupts the freezing process. at what temperature does salt melt ice? that upsets the balance; At a temperature of 30. What Temp Does Salt Melt Ice Celsius.
From courses.lumenlearning.com
Phase Change and Latent Heat Physics What Temp Does Salt Melt Ice Celsius pure water freezes at 32°f (0°c). when salt is applied to ice, it disrupts the freezing process. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. But how does salt do it? First, it’s important to understand a bit about h 2 o in the winter. Water with. What Temp Does Salt Melt Ice Celsius.
From quizdbbarnstorms.z21.web.core.windows.net
What Temperature Does Ice Melt In Celsius What Temp Does Salt Melt Ice Celsius at what temperature does salt melt ice? But how does salt do it? Water with salt (or any other substance in it) will freeze at some lower temperature. More water is melting than freezing at the normal freezing temperature, and so the ice. This happens because salt lowers the freezing point of water, causing the ice to melt at. What Temp Does Salt Melt Ice Celsius.
From safepaw.com
What Temperature Does Salt Melt Ice? Science Behind Salt and Ice What Temp Does Salt Melt Ice Celsius when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. that upsets the balance; pure water freezes at 32°f (0°c). Water with salt (or any other substance in it) will freeze at some lower temperature. More water is melting than freezing at the normal freezing. What Temp Does Salt Melt Ice Celsius.
From www.livescience.com
Kelvin Temperature Scale Facts and History Live Science What Temp Does Salt Melt Ice Celsius But how does salt do it? pure water freezes at 32°f (0°c). This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. when salt is applied to ice, it disrupts the freezing process. First, it’s important to. What Temp Does Salt Melt Ice Celsius.
From chem.libretexts.org
10.4 Phase Diagrams Chemistry LibreTexts What Temp Does Salt Melt Ice Celsius when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. at what temperature does salt melt ice? Water with salt (or any other substance in it) will freeze at some lower temperature. More water is melting than freezing at the normal freezing temperature, and so the. What Temp Does Salt Melt Ice Celsius.
From quizguarantors.z4.web.core.windows.net
What Temperature Does Ice Melt In Celsius What Temp Does Salt Melt Ice Celsius Water with salt (or any other substance in it) will freeze at some lower temperature. But how does salt do it? First, it’s important to understand a bit about h 2 o in the winter. at what temperature does salt melt ice? when salt is applied to ice, it disrupts the freezing process. More water is melting than. What Temp Does Salt Melt Ice Celsius.
From www.britannica.com
Ice Definition, Structure, Properties, Freezing Point, & Facts What Temp Does Salt Melt Ice Celsius that upsets the balance; when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. when salt is applied to ice, it disrupts the freezing process. at what temperature does salt melt ice? pure water freezes at 32°f (0°c). First, it’s important to understand. What Temp Does Salt Melt Ice Celsius.
From studyzonepatristic.z13.web.core.windows.net
Why Does Adding Salt To Ice Make It Melt What Temp Does Salt Melt Ice Celsius At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. at what temperature does salt melt ice? when. What Temp Does Salt Melt Ice Celsius.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) What Temp Does Salt Melt Ice Celsius at what temperature does salt melt ice? At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. More water is melting than freezing at the normal freezing. What Temp Does Salt Melt Ice Celsius.
From laptrinhx.com
How does salt melt snow? LaptrinhX / News What Temp Does Salt Melt Ice Celsius that upsets the balance; More water is melting than freezing at the normal freezing temperature, and so the ice. more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0. What Temp Does Salt Melt Ice Celsius.
From dxotswbpt.blob.core.windows.net
Will Salt Water Melt Ice at Jimmy Chang blog What Temp Does Salt Melt Ice Celsius But how does salt do it? when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point.. What Temp Does Salt Melt Ice Celsius.
From dengarden.com
The Difference Between Rock Salt and Ice Melt Dengarden What Temp Does Salt Melt Ice Celsius when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. Water with salt (or any other substance in it) will freeze at some lower temperature. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. pure water. What Temp Does Salt Melt Ice Celsius.
From waterseer.org
Does Water Softening Salt Melt Ice? Everything You Need to Know What Temp Does Salt Melt Ice Celsius when salt is applied to ice, it disrupts the freezing process. But how does salt do it? pure water freezes at 32°f (0°c). More water is melting than freezing at the normal freezing temperature, and so the ice. that upsets the balance; at what temperature does salt melt ice? At a temperature of 30 degrees (f),. What Temp Does Salt Melt Ice Celsius.
From www.pinterest.com
Candy Making Temperature Chart Temperature chart, Candy making What Temp Does Salt Melt Ice Celsius that upsets the balance; But how does salt do it? More water is melting than freezing at the normal freezing temperature, and so the ice. First, it’s important to understand a bit about h 2 o in the winter. at what temperature does salt melt ice? when salt is added to ice, it dissolves into the water. What Temp Does Salt Melt Ice Celsius.
From xecogioinhapkhau.com
Does Salt Melt Easily When Heated? Exploring Its Melting Point What Temp Does Salt Melt Ice Celsius more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. at what temperature does salt melt ice? when salt is added to ice, it dissolves into the water present on the ice's surface, creating a solution with a lower. This happens because salt lowers the freezing point. What Temp Does Salt Melt Ice Celsius.
From www.pinterest.co.uk
Will Epsom Salt Melt Ice? And What Else Can Melt Ice? Ice melting What Temp Does Salt Melt Ice Celsius when salt is applied to ice, it disrupts the freezing process. First, it’s important to understand a bit about h 2 o in the winter. more than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. More water is melting than freezing at the normal freezing temperature, and so. What Temp Does Salt Melt Ice Celsius.
From waterseer.org
Does Water Softening Salt Melt Ice? Everything You Need to Know What Temp Does Salt Melt Ice Celsius that upsets the balance; This happens because salt lowers the freezing point of water, causing the ice to melt at temperatures below 0 degrees celsius or 32 degrees fahrenheit, which is water’s normal freezing point. pure water freezes at 32°f (0°c). at what temperature does salt melt ice? when salt is applied to ice, it disrupts. What Temp Does Salt Melt Ice Celsius.
From meltsnow.com
Salt vs. Temperature What Temp Does Salt Melt Ice Celsius First, it’s important to understand a bit about h 2 o in the winter. At a temperature of 30 degrees (f), one pound of salt (sodium chloride) will melt 46 pounds of ice. But how does salt do it? that upsets the balance; This happens because salt lowers the freezing point of water, causing the ice to melt at. What Temp Does Salt Melt Ice Celsius.