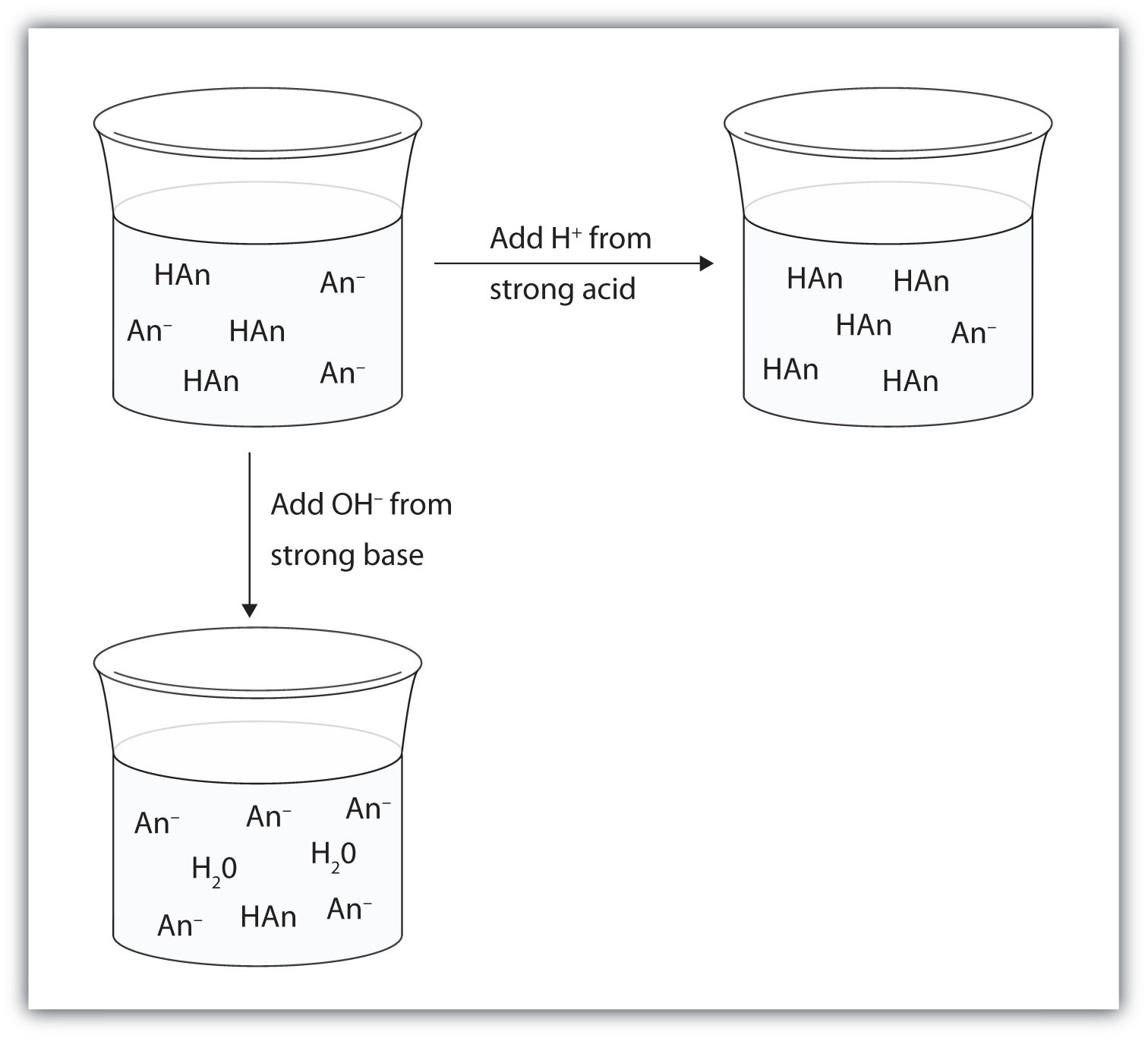
Buffer Capacity Definition Biology . qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. to review, buffer solutions contain a weak acid and its conjugate base. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. In practice, a buffer solution contains. buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. Buffers that have more solute dissolved in them to start with have larger capacities,. a buffer system is a solution that resists a change in ph when acids or bases are added to it. The ph of a solution is a measure. The ph scale ranges from 0 to 14. we say that a buffer has a certain capacity. They have maximal buffering capacity at a ph = pka. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. define buffers and discuss the role they play in human biology.
from courses.lumenlearning.com
The ph scale ranges from 0 to 14. The ph of a solution is a measure. They have maximal buffering capacity at a ph = pka. define buffers and discuss the role they play in human biology. we say that a buffer has a certain capacity. buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. Buffers that have more solute dissolved in them to start with have larger capacities,. In practice, a buffer solution contains. a buffer system is a solution that resists a change in ph when acids or bases are added to it. A buffer is an aqueous solution used to keep the ph of a solution nearly constant.
10.5 Buffers The Basics of General, Organic, and Biological Chemistry
Buffer Capacity Definition Biology In practice, a buffer solution contains. They have maximal buffering capacity at a ph = pka. define buffers and discuss the role they play in human biology. to review, buffer solutions contain a weak acid and its conjugate base. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. In practice, a buffer solution contains. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. a buffer system is a solution that resists a change in ph when acids or bases are added to it. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. we say that a buffer has a certain capacity. The ph of a solution is a measure. The ph scale ranges from 0 to 14. Buffers that have more solute dissolved in them to start with have larger capacities,. buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit.
From ar.inspiredpencil.com
Buffer Definition Buffer Capacity Definition Biology Buffers that have more solute dissolved in them to start with have larger capacities,. The ph scale ranges from 0 to 14. a buffer system is a solution that resists a change in ph when acids or bases are added to it. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added. Buffer Capacity Definition Biology.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Buffer Capacity Definition Biology The ph scale ranges from 0 to 14. we say that a buffer has a certain capacity. The ph of a solution is a measure. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. a buffer system is a solution that resists a change in ph. Buffer Capacity Definition Biology.
From www.brainkart.com
Buffers Buffer Capacity Definition Biology A buffer is an aqueous solution used to keep the ph of a solution nearly constant. The ph of a solution is a measure. In practice, a buffer solution contains. we say that a buffer has a certain capacity. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. define. Buffer Capacity Definition Biology.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Buffer Capacity Definition Biology A buffer is an aqueous solution used to keep the ph of a solution nearly constant. In practice, a buffer solution contains. buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. They have maximal buffering capacity at a. Buffer Capacity Definition Biology.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Buffer Capacity Definition Biology buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. The ph of a solution is a measure. to review, buffer solutions contain a weak acid and its conjugate base. define buffers and discuss the role they. Buffer Capacity Definition Biology.
From courses.lumenlearning.com
10.5 Buffers The Basics of General, Organic, and Biological Chemistry Buffer Capacity Definition Biology qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. The ph scale ranges from 0 to 14. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. define buffers and discuss the role they play in human biology. The ph of. Buffer Capacity Definition Biology.
From www.slideserve.com
PPT Acids and bases, pH and buffers PowerPoint Presentation, free Buffer Capacity Definition Biology a buffer system is a solution that resists a change in ph when acids or bases are added to it. define buffers and discuss the role they play in human biology. to review, buffer solutions contain a weak acid and its conjugate base. In practice, a buffer solution contains. Buffer capacity is a quantitative measure of the. Buffer Capacity Definition Biology.
From www.pinterest.co.uk
Buffer Easy Science Study chemistry, Chemistry classroom, Organic Buffer Capacity Definition Biology qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. define buffers and discuss the role they play in human biology. a buffer system is a solution that resists a change. Buffer Capacity Definition Biology.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Buffer Capacity Definition Biology define buffers and discuss the role they play in human biology. In practice, a buffer solution contains. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. Buffers that have more solute dissolved in them to start with have larger capacities,. They have maximal buffering capacity at a. Buffer Capacity Definition Biology.
From facts.net
13 Enigmatic Facts About Buffer Capacity Buffer Capacity Definition Biology A buffer is an aqueous solution used to keep the ph of a solution nearly constant. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. a buffer system is a solution that resists a change in ph when acids or bases are added to it. Buffers that have more solute. Buffer Capacity Definition Biology.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Buffer Capacity Definition Biology The ph of a solution is a measure. buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. a buffer system is a solution that resists a change in ph when acids or bases are added to it.. Buffer Capacity Definition Biology.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure Buffer Capacity Definition Biology define buffers and discuss the role they play in human biology. we say that a buffer has a certain capacity. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. They have maximal buffering capacity at a ph = pka. The ph of a solution is a measure. Buffer capacity is a. Buffer Capacity Definition Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Capacity Definition Biology The ph of a solution is a measure. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. Buffers that have more solute dissolved in them to start with have larger capacities,. In practice, a buffer solution contains. define buffers and discuss the role they play in human biology. to. Buffer Capacity Definition Biology.
From www.youtube.com
Acid, Base & Buffer in Biological systems YouTube Buffer Capacity Definition Biology to review, buffer solutions contain a weak acid and its conjugate base. buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be. Buffer Capacity Definition Biology.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Buffer Capacity Definition Biology In practice, a buffer solution contains. They have maximal buffering capacity at a ph = pka. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. Buffers that have more solute dissolved in them to start with have larger capacities,. we say that a buffer has a certain capacity. The ph. Buffer Capacity Definition Biology.
From www.youtube.com
Buffers definition and the role of buffer in the body YouTube Buffer Capacity Definition Biology buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. The ph scale ranges from 0 to 14. we say that a buffer has a certain capacity. Buffer capacity is a quantitative measure of the resistance to change. Buffer Capacity Definition Biology.
From gamesmartz.com
Buffer Capacity Definition & Image GameSmartz Buffer Capacity Definition Biology to review, buffer solutions contain a weak acid and its conjugate base. The ph scale ranges from 0 to 14. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. define buffers and discuss the role they play in human biology. a buffer system is a solution that resists a change. Buffer Capacity Definition Biology.
From www.youtube.com
Buffers and pH Biology YouTube Buffer Capacity Definition Biology buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. we say that a buffer has a certain capacity. A. Buffer Capacity Definition Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer Capacity Definition Biology qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. In practice, a buffer solution contains. we say that a buffer has a certain capacity. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. They have maximal buffering capacity. Buffer Capacity Definition Biology.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Buffer Capacity Definition Biology Buffers that have more solute dissolved in them to start with have larger capacities,. we say that a buffer has a certain capacity. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. They have maximal buffering capacity at a ph = pka. In practice, a buffer solution. Buffer Capacity Definition Biology.
From www.youtube.com
Buffer Capacity YouTube Buffer Capacity Definition Biology qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. define buffers and discuss the role they play in human biology. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. Buffers that have more solute dissolved in them to start with. Buffer Capacity Definition Biology.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Capacity Definition Biology qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. The ph scale ranges from 0 to 14. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. The ph of a solution is a measure. They have maximal buffering capacity. Buffer Capacity Definition Biology.
From www.slideserve.com
PPT Chapter Two Water The Solvent for Biochemical Reactions Buffer Capacity Definition Biology Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. to review, buffer solutions contain a weak acid and its conjugate base. The ph of a solution is a measure. They have maximal buffering capacity at a ph = pka. buffer capacity is defined as the number of moles of. Buffer Capacity Definition Biology.
From www.slideshare.net
Buffer system Buffer Capacity Definition Biology A buffer is an aqueous solution used to keep the ph of a solution nearly constant. a buffer system is a solution that resists a change in ph when acids or bases are added to it. we say that a buffer has a certain capacity. qualitatively, buffering capacity can be defined as the amount of strong acid/base. Buffer Capacity Definition Biology.
From www.numerade.com
SOLVEDDefine buffer capacity. Buffer Capacity Definition Biology we say that a buffer has a certain capacity. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. In practice, a buffer solution contains. The ph scale ranges from 0 to. Buffer Capacity Definition Biology.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Buffer Capacity Definition Biology define buffers and discuss the role they play in human biology. Buffers that have more solute dissolved in them to start with have larger capacities,. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. we say that a buffer has a certain capacity. The ph of. Buffer Capacity Definition Biology.
From anatomyzonegarrett88.z5.web.core.windows.net
respiratory buffer system Buffer Capacity Definition Biology The ph of a solution is a measure. we say that a buffer has a certain capacity. a buffer system is a solution that resists a change in ph when acids or bases are added to it. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution.. Buffer Capacity Definition Biology.
From www.slideserve.com
PPT 5. Buffering capacity PowerPoint Presentation, free download ID Buffer Capacity Definition Biology to review, buffer solutions contain a weak acid and its conjugate base. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph. Buffer Capacity Definition Biology.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems Buffer Capacity Definition Biology Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. we say that a buffer has a certain capacity. The ph scale ranges from 0 to 14. The ph of a solution is a measure. A buffer is an aqueous solution used to keep the ph of a solution nearly constant.. Buffer Capacity Definition Biology.
From ruslan179144.blogspot.com
Define Definition Of Buffer Capacity Robert Romero Coiffure Buffer Capacity Definition Biology a buffer system is a solution that resists a change in ph when acids or bases are added to it. The ph scale ranges from 0 to 14. The ph of a solution is a measure. qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. we. Buffer Capacity Definition Biology.
From www.expii.com
Buffers — Definition & Overview Expii Buffer Capacity Definition Biology qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution. define buffers and discuss the role they play in human biology. to review, buffer solutions contain a weak acid and its conjugate base. A buffer is an aqueous solution used to keep the ph of a solution. Buffer Capacity Definition Biology.
From users.highland.edu
Buffers Buffer Capacity Definition Biology a buffer system is a solution that resists a change in ph when acids or bases are added to it. The ph of a solution is a measure. we say that a buffer has a certain capacity. The ph scale ranges from 0 to 14. to review, buffer solutions contain a weak acid and its conjugate base.. Buffer Capacity Definition Biology.
From www.onlinebiologynotes.com
Buffer, buffering capacity, properties of good buffer and role of Buffer Capacity Definition Biology to review, buffer solutions contain a weak acid and its conjugate base. In practice, a buffer solution contains. They have maximal buffering capacity at a ph = pka. we say that a buffer has a certain capacity. buffer capacity is defined as the number of moles of acid or base that have to be added to 1. Buffer Capacity Definition Biology.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 Buffer Capacity Definition Biology buffer capacity is defined as the number of moles of acid or base that have to be added to 1 liter to cause its ph to change by 1 unit. Buffers that have more solute dissolved in them to start with have larger capacities,. They have maximal buffering capacity at a ph = pka. Buffer capacity is a quantitative. Buffer Capacity Definition Biology.
From users.highland.edu
Buffers Buffer Capacity Definition Biology we say that a buffer has a certain capacity. Buffer capacity is a quantitative measure of the resistance to change of ph of a solution containing a. define buffers and discuss the role they play in human biology. In practice, a buffer solution contains. The ph of a solution is a measure. The ph scale ranges from 0. Buffer Capacity Definition Biology.