Endothermic Exothermic Solution Process . an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. In this investigation, students classify chemical reactions as exothermic or endothermic. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. State the law of conservation of energy. If δh is negative, the. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic.
from www.clutchprep.com
State the law of conservation of energy. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. In this investigation, students classify chemical reactions as exothermic or endothermic. If δh is negative, the. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic.
Endothermic & Exothermic Reactions Chemistry Video Clutch Prep
Endothermic Exothermic Solution Process photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. If δh is negative, the. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. In this investigation, students classify chemical reactions as exothermic or endothermic. State the law of conservation of energy. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat.
From catholicscienceteacher8.blogspot.com
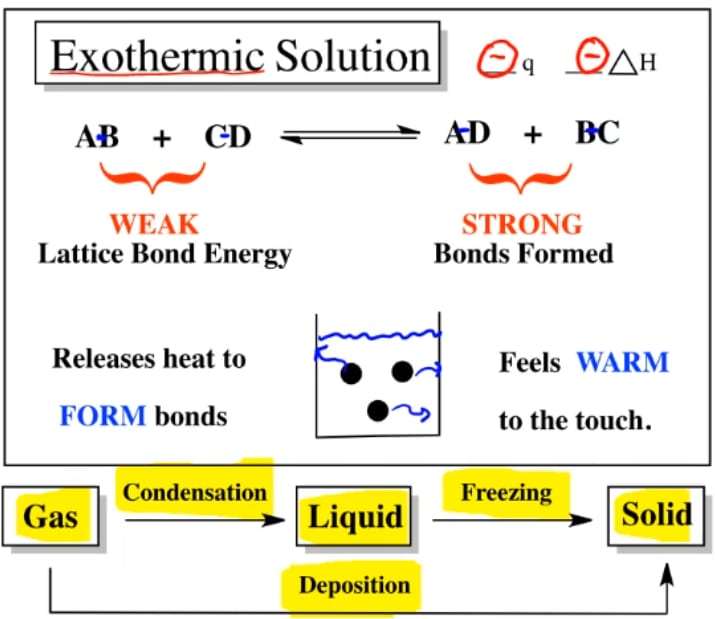
Mrs. Remis' Science Blog 8th grade ENDOTHERMIC & EXOTHERMIC PROCESS Endothermic Exothermic Solution Process if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. If δh is negative,. Endothermic Exothermic Solution Process.
From mungfali.com
Exothermic Vs Endothermic Diagram Endothermic Exothermic Solution Process In this investigation, students classify chemical reactions as exothermic or endothermic. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. endothermic dissolutions require greater energy input to separate the solute. Endothermic Exothermic Solution Process.
From www.toppr.com
Give an example each of an exothermic an endothermic process. Endothermic Exothermic Solution Process if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. If δh is negative, the. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. In this investigation,. Endothermic Exothermic Solution Process.
From www.chegg.com
Solved Part A Identify whether each process is endothermic Endothermic Exothermic Solution Process endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. State the law of conservation. Endothermic Exothermic Solution Process.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Exothermic Solution Process In this investigation, students classify chemical reactions as exothermic or endothermic. If δh is negative, the. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. an exothermic process releases heat, causing the. Endothermic Exothermic Solution Process.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Endothermic Exothermic Solution Process If δh is negative, the. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. In this investigation, students. Endothermic Exothermic Solution Process.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Exothermic Solution Process State the law of conservation of energy. In this investigation, students classify chemical reactions as exothermic or endothermic. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. If δh is negative, the. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an. Endothermic Exothermic Solution Process.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Endothermic Exothermic Solution Process State the law of conservation of energy. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. In this investigation, students classify chemical reactions as exothermic or endothermic. If δh is negative, the. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are.. Endothermic Exothermic Solution Process.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Exothermic Solution Process photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. for example, the solution of. Endothermic Exothermic Solution Process.
From www.bigstockphoto.com
Endothermic Exothermic Image & Photo (Free Trial) Bigstock Endothermic Exothermic Solution Process an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. State the law of conservation of energy. If δh is negative, the. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. if δh is positive, the process absorbs heat from the surroundings and is said. Endothermic Exothermic Solution Process.
From deangelokruwvalencia.blogspot.com
Determine Whether the Process Is Endothermic or Exothermic Endothermic Exothermic Solution Process solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. If δh is negative, the. State the law of conservation of energy. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar. Endothermic Exothermic Solution Process.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Exothermic Solution Process State the law of conservation of energy. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. In this investigation, students classify chemical reactions as exothermic or endothermic. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. if δh is positive, the process absorbs. Endothermic Exothermic Solution Process.
From www.numerade.com
SOLVED Use thc Exothermic and Endothermic intcractive t0 classify the Endothermic Exothermic Solution Process if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. endothermic dissolutions require greater energy. Endothermic Exothermic Solution Process.
From techiescientist.com
Is Condensation Endothermic or Exothermic? Techiescientist Endothermic Exothermic Solution Process If δh is negative, the. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. In this investigation, students classify chemical reactions as exothermic or endothermic. endothermic dissolutions require greater energy. Endothermic Exothermic Solution Process.
From mavink.com
Exothermic Reaction Class 10 Endothermic Exothermic Solution Process If δh is negative, the. State the law of conservation of energy. In this investigation, students classify chemical reactions as exothermic or endothermic. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. solvation can be an exothermic or endothermic process depending on the nature of the solute and. Endothermic Exothermic Solution Process.
From baekhyun.netlify.app
What Is Exothermic Endothermic Exothermic Solution Process In this investigation, students classify chemical reactions as exothermic or endothermic. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. If δh is negative, the. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. State the law of conservation of energy. endothermic. Endothermic Exothermic Solution Process.
From www.youtube.com
Why are some solution processes exothermic whereas others are Endothermic Exothermic Solution Process an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. endothermic dissolutions require greater energy input to separate. Endothermic Exothermic Solution Process.
From mungfali.com
Exothermic And Endothermic Chemical Reactions Endothermic Exothermic Solution Process endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. In this investigation, students classify chemical reactions as exothermic or endothermic. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. State the law of conservation of energy. solvation can be an exothermic or endothermic. Endothermic Exothermic Solution Process.
From www.vrogue.co
Exothermic Vs Endothermic Reaction Graphs Energy Acti vrogue.co Endothermic Exothermic Solution Process State the law of conservation of energy. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and. Endothermic Exothermic Solution Process.
From alwafd.org
Endothermic Reaction Definition, Equation, Graph & Examples What Endothermic Exothermic Solution Process If δh is negative, the. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the. Endothermic Exothermic Solution Process.
From study.com
Endothermic vs. Exothermic Reactions Process & Examples Video Endothermic Exothermic Solution Process if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. In this investigation, students classify chemical reactions as exothermic. Endothermic Exothermic Solution Process.
From www.animalia-life.club
Endothermic And Exothermic Reaction Graph Endothermic Exothermic Solution Process State the law of conservation of energy. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. In this investigation, students classify chemical reactions as exothermic or endothermic. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. endothermic dissolutions require greater energy. Endothermic Exothermic Solution Process.
From quizizz.com
Endothermic vs Exothermic Process Chemistry Quiz Quizizz Endothermic Exothermic Solution Process an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. solvation can be an exothermic. Endothermic Exothermic Solution Process.
From www.myxxgirl.com
Exothermic And Endothermic Processes My XXX Hot Girl Endothermic Exothermic Solution Process photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. for example, the solution of sodium. Endothermic Exothermic Solution Process.
From pressbooks.online.ucf.edu
12.1 The Dissolution Process Chemistry Fundamentals Endothermic Exothermic Solution Process solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. In this investigation, students classify chemical reactions as exothermic or endothermic. State the law of conservation of energy. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. if δh is positive,. Endothermic Exothermic Solution Process.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Exothermic Solution Process State the law of conservation of energy. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. If δh is negative, the. photosynthesis, the process that allows plants to convert carbon dioxide and. Endothermic Exothermic Solution Process.
From saylordotorg.github.io
Factors Affecting Solution Formation Endothermic Exothermic Solution Process If δh is negative, the. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. In this investigation, students classify chemical reactions as exothermic or endothermic. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. State the law of conservation of. Endothermic Exothermic Solution Process.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Exothermic Solution Process for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. State the law of conservation of energy. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. . Endothermic Exothermic Solution Process.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Exothermic Solution Process endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. solvation can be an exothermic or endothermic. Endothermic Exothermic Solution Process.
From www.youtube.com
31 Endothermic and Exothermic Processes Solutions YouTube Endothermic Exothermic Solution Process In this investigation, students classify chemical reactions as exothermic or endothermic. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. State the law of conservation of energy. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. endothermic dissolutions require greater energy input. Endothermic Exothermic Solution Process.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Endothermic Exothermic Solution Process In this investigation, students classify chemical reactions as exothermic or endothermic. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. for example, the solution of sodium hydroxide is exothermic, and the solution of. Endothermic Exothermic Solution Process.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Exothermic Solution Process for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. In this investigation, students classify chemical reactions as exothermic or endothermic. If δh is negative, the. State the law of conservation of energy. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. photosynthesis, the process. Endothermic Exothermic Solution Process.
From www.clutchprep.com
Endothermic & Exothermic Reactions Chemistry Video Clutch Prep Endothermic Exothermic Solution Process State the law of conservation of energy. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise.. Endothermic Exothermic Solution Process.
From www.researchgate.net
Endothermic or exothermic reaction of the VO 2 phase caused by phase Endothermic Exothermic Solution Process If δh is negative, the. for example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat. solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. endothermic dissolutions require greater energy input to separate the solute species than is recovered when the solutes are.. Endothermic Exothermic Solution Process.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Exothermic Solution Process an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. photosynthesis, the process that allows plants to convert carbon dioxide and water to sugar and oxygen, is an endothermic. if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. In this investigation, students classify chemical reactions. Endothermic Exothermic Solution Process.