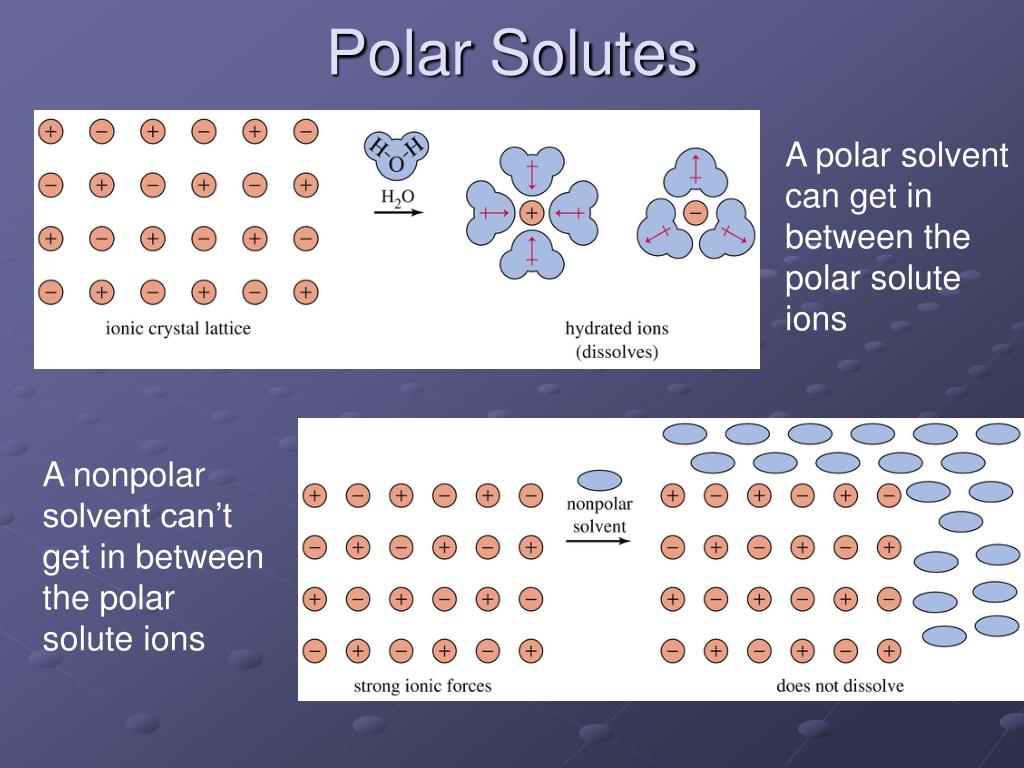
What Are Some Examples Of Polar Solvent . Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. For example, water is a molecule. It can serve two major purposes: Would i 2 be more soluble in ccl 4 or h 2 o? Water is a polar solvent; A solvent is a liquid that serves as the medium for a reaction. 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: Solvents used in organic chemistry are characterized by their physical characteristics. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial.
from www.slideserve.com
Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. For example, water is a molecule. Water is a polar solvent; A solvent is a liquid that serves as the medium for a reaction. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: It can serve two major purposes: Would i 2 be more soluble in ccl 4 or h 2 o? Solvents used in organic chemistry are characterized by their physical characteristics. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape.
PPT Solutions PowerPoint Presentation, free download ID3746599
What Are Some Examples Of Polar Solvent A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: It can serve two major purposes: Solvents used in organic chemistry are characterized by their physical characteristics. A solvent is a liquid that serves as the medium for a reaction. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. Would i 2 be more soluble in ccl 4 or h 2 o? Water is a polar solvent; For example, water is a molecule. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial.
From maryi-pubic.blogspot.com
Polar Aprotic Solvents Meaning / Nucleophilic Substitution And What Are Some Examples Of Polar Solvent Water is a polar solvent; Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: It can serve two major purposes: Solvents used in organic chemistry are characterized by their physical characteristics. A solvent. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID What Are Some Examples Of Polar Solvent It can serve two major purposes: For example, water is a molecule. 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: Solvents used in organic chemistry are characterized by their physical characteristics. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Polar solvent molecules have. What Are Some Examples Of Polar Solvent.
From byjus.com
Electrovalent compounds have low melting point low boiling point What Are Some Examples Of Polar Solvent Solvents used in organic chemistry are characterized by their physical characteristics. It can serve two major purposes: For example, water is a molecule. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: Water is a polar solvent;. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT 11. Reactions of Alkyl Halides Nucleophilic Substitutions and What Are Some Examples Of Polar Solvent A solvent is a liquid that serves as the medium for a reaction. Water is a polar solvent; 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. For example, water is a molecule.. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2674214 What Are Some Examples Of Polar Solvent Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. A solvent is a liquid that serves as the medium for a reaction. Solvents used in organic chemistry are characterized by their physical characteristics. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. 23 rows two important numbers that. What Are Some Examples Of Polar Solvent.
From www.slideshare.net
Podcastppt5 What Are Some Examples Of Polar Solvent Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. It can serve two major purposes: Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. A solvent is a liquid that serves as the medium for a reaction. Solvents used in. What Are Some Examples Of Polar Solvent.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts What Are Some Examples Of Polar Solvent A solvent is a liquid that serves as the medium for a reaction. Water is a polar solvent; It can serve two major purposes: For example, water is a molecule. 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: Solvents used in organic chemistry are characterized by their physical characteristics. Polar solvent molecules have. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Topic 12 Solutions PowerPoint Presentation, free download ID What Are Some Examples Of Polar Solvent Would i 2 be more soluble in ccl 4 or h 2 o? Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. Water is a polar solvent; A solvent is a liquid that serves as the medium for a reaction. Polar solvent molecules have an asymmetrical distribution of electron density, and some. What Are Some Examples Of Polar Solvent.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Master What Are Some Examples Of Polar Solvent It can serve two major purposes: For example, water is a molecule. Solvents used in organic chemistry are characterized by their physical characteristics. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. Would i 2 be more soluble in ccl 4 or h 2 o? Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a. What Are Some Examples Of Polar Solvent.
From brainly.in
What is a polar solvent ? Brainly.in What Are Some Examples Of Polar Solvent A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 What Are Some Examples Of Polar Solvent A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. Solvents used in organic chemistry are characterized by their physical characteristics. It can serve two major purposes: 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: Polar. What Are Some Examples Of Polar Solvent.
From www.researchgate.net
Solvent properties of some polar aprotic solvents (Xu, 2004) Download What Are Some Examples Of Polar Solvent Solvents used in organic chemistry are characterized by their physical characteristics. Water is a polar solvent; For example, water is a molecule. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: It can serve two major purposes:. What Are Some Examples Of Polar Solvent.
From stilleducation.com
Polar and Nonpolar Covalent Bonds Characteristics & Differences What Are Some Examples Of Polar Solvent For example, water is a molecule. It can serve two major purposes: Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. Would i 2 be more soluble in ccl 4 or h 2 o? Water is a polar solvent; Solvents used in organic chemistry are characterized by their physical characteristics. 23 rows. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Alkyl halides PowerPoint Presentation, free download ID599053 What Are Some Examples Of Polar Solvent A solvent is a liquid that serves as the medium for a reaction. For example, water is a molecule. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. Solvents used in. What Are Some Examples Of Polar Solvent.
From www.researchgate.net
Photographs showing F8BTPEG solubility in polar solvents (1 mg mL −1 What Are Some Examples Of Polar Solvent A solvent is a liquid that serves as the medium for a reaction. Would i 2 be more soluble in ccl 4 or h 2 o? Water is a polar solvent; For example, water is a molecule. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. It can serve two major purposes:. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3746599 What Are Some Examples Of Polar Solvent Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. For example, water is a molecule. Solvents used in organic chemistry are characterized by their physical characteristics. A solvent is a liquid that serves as the medium for a reaction. A polar solvent is. What Are Some Examples Of Polar Solvent.
From slideplayer.com
Chapter 2 Chemistry ppt download What Are Some Examples Of Polar Solvent A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. It can serve two major purposes: A solvent is a liquid that serves as the medium for a reaction. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. Solvents used. What Are Some Examples Of Polar Solvent.
From www.youtube.com
What are examples of polar solvents? YouTube What Are Some Examples Of Polar Solvent For example, water is a molecule. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. Would i 2 be more soluble in ccl 4 or h 2 o? A polar solvent. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID375520 What Are Some Examples Of Polar Solvent For example, water is a molecule. A solvent is a liquid that serves as the medium for a reaction. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Solvents used in organic chemistry are characterized by their physical characteristics. Water is a polar solvent; Would i 2 be more soluble in. What Are Some Examples Of Polar Solvent.
From www.chemistryworld.com
Polar solvents promote halogen bonds over hydrogen ones Research What Are Some Examples Of Polar Solvent Water is a polar solvent; Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. Solvents used in organic chemistry are characterized by their physical characteristics. For example, water is a molecule. Would i 2 be more soluble in ccl 4 or h 2 o? A polar solvent is a liquid with molecules that have a slight electrical charge due to. What Are Some Examples Of Polar Solvent.
From www.youtube.com
Chapter 08 05 Water A Polar Solvent YouTube What Are Some Examples Of Polar Solvent Water is a polar solvent; 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: Would i 2 be more soluble in ccl 4 or h 2 o? Solvents used in organic chemistry are characterized by their physical characteristics. It can serve two major purposes: Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. A. What Are Some Examples Of Polar Solvent.
From printableliblibated.z21.web.core.windows.net
Water As A Universal Solvent Experiment What Are Some Examples Of Polar Solvent Would i 2 be more soluble in ccl 4 or h 2 o? 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: For example, water is a molecule. Solvents used in organic chemistry are characterized by their physical characteristics. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. A polar solvent is a liquid. What Are Some Examples Of Polar Solvent.
From mungfali.com
Polar Protic Solvent Examples What Are Some Examples Of Polar Solvent Solvents used in organic chemistry are characterized by their physical characteristics. For example, water is a molecule. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Water is a polar solvent; Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID375520 What Are Some Examples Of Polar Solvent For example, water is a molecule. A solvent is a liquid that serves as the medium for a reaction. Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. Other polar solvents. What Are Some Examples Of Polar Solvent.
From mungfali.com
Solvent Polarity Chart What Are Some Examples Of Polar Solvent 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: A solvent is a liquid that serves as the medium for a reaction. Water is a polar solvent; A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. For example, water is a molecule. Polar protic solvents. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 What Are Some Examples Of Polar Solvent Solvents used in organic chemistry are characterized by their physical characteristics. Water is a polar solvent; A solvent is a liquid that serves as the medium for a reaction. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Would i 2 be. What Are Some Examples Of Polar Solvent.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Master What Are Some Examples Of Polar Solvent Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. A solvent is a liquid that serves as the medium for a reaction. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. It can serve two major purposes: For example, water is a molecule. Water is a polar solvent;. What Are Some Examples Of Polar Solvent.
From www.thoughtco.com
Definition and Examples of a Polar Bond What Are Some Examples Of Polar Solvent Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. Water is a polar solvent; Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. It can serve two major purposes: A polar solvent is a liquid with molecules that have a. What Are Some Examples Of Polar Solvent.
From slideplayer.com
UNIT 6 Solution Chemistry. ppt download What Are Some Examples Of Polar Solvent For example, water is a molecule. A solvent is a liquid that serves as the medium for a reaction. Solvents used in organic chemistry are characterized by their physical characteristics. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Polar solvent molecules have an asymmetrical distribution of electron density, and some. What Are Some Examples Of Polar Solvent.
From techblog.ctgclean.com
Chemistry Solvent Characteristics CTG Clean What Are Some Examples Of Polar Solvent A solvent is a liquid that serves as the medium for a reaction. Solvents used in organic chemistry are characterized by their physical characteristics. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. For example, water is a molecule. 23 rows two important numbers that are helpful in identifying a solvent's. What Are Some Examples Of Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3746599 What Are Some Examples Of Polar Solvent Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. For example, water is a molecule. It can serve two major purposes: Water is a polar solvent; Solvents used in organic chemistry are characterized by their physical characteristics. A solvent is a liquid that serves as the medium for a reaction. Would i 2 be more soluble in ccl 4 or. What Are Some Examples Of Polar Solvent.
From silviaq-bettor.blogspot.com
Solvents For Sn2 Reactions Ppt Substitution And Elimination Reactions What Are Some Examples Of Polar Solvent 23 rows two important numbers that are helpful in identifying a solvent's polarity are its: It can serve two major purposes: Water is a polar solvent; For example, water is a molecule. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Polar. What Are Some Examples Of Polar Solvent.
From tutors.com
What is a solvent? Definition & Examples (Video) What Are Some Examples Of Polar Solvent Would i 2 be more soluble in ccl 4 or h 2 o? Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. A polar solvent is a liquid with molecules that have a slight electrical charge due to its shape. Polar solvent molecules. What Are Some Examples Of Polar Solvent.
From chemawareness.com
Classification of Solvents Chem Awareness What Are Some Examples Of Polar Solvent Water is a polar solvent; Polar solvent molecules have an asymmetrical distribution of electron density, and some areas of a polar solvent molecule carry a partial. Would i 2 be more soluble in ccl 4 or h 2 o? Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. A polar solvent is a liquid with molecules that have a slight. What Are Some Examples Of Polar Solvent.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents What Are Some Examples Of Polar Solvent Polar protic solvents include acetic acid, methanol and ethanol, while aprotic solvents include ethyl acetate and tetrahydrofuran. It can serve two major purposes: Solvents used in organic chemistry are characterized by their physical characteristics. Other polar solvents include acetone, acetonitrile, dimethylformamide (dmf), dimelthylsulfoxide. For example, water is a molecule. Would i 2 be more soluble in ccl 4 or h. What Are Some Examples Of Polar Solvent.