Copper(Ii) Sulfate And Sodium Hydroxide Precipitate . How many grams of zinc(ii) nitrate and sodium sulfide. Enter an equation of an ionic chemical equation and press the balance button. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: The balanced equation will be calculated along with the. Sodium hydroxide with copper (ii) sulfate. Here, copper (ii) sulfate (cuso 4) is added to sodium. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Sodium chloride, sodium hydroxide, or sodium sulfate?. Your browser does not support the video tag. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate.
from stock.adobe.com
How many grams of zinc(ii) nitrate and sodium sulfide. Enter an equation of an ionic chemical equation and press the balance button. Here, copper (ii) sulfate (cuso 4) is added to sodium. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. Your browser does not support the video tag. Sodium chloride, sodium hydroxide, or sodium sulfate?. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium hydroxide with copper (ii) sulfate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate.
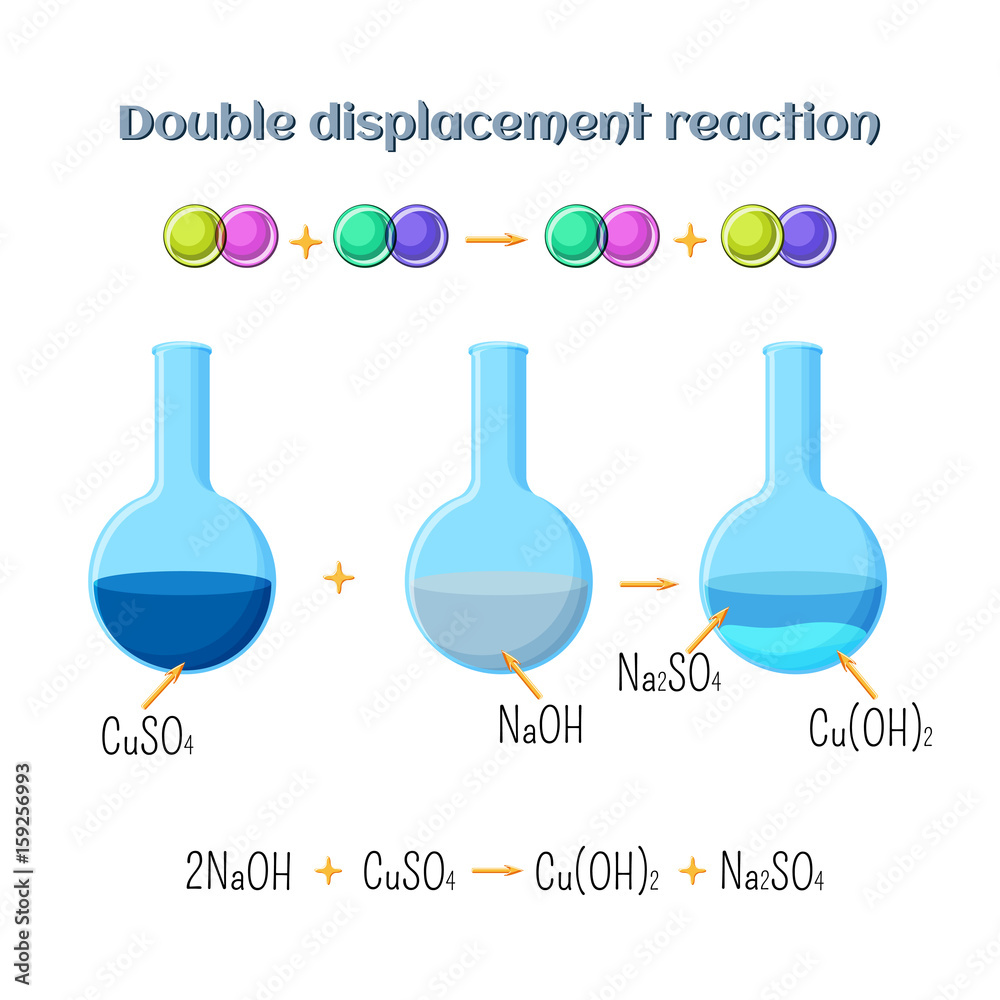
Double displacement reaction sodium hydroxide and copper sulfate. Types of chemical reactions
Copper(Ii) Sulfate And Sodium Hydroxide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium hydroxide with copper (ii) sulfate. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Sodium chloride, sodium hydroxide, or sodium sulfate?. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Here, copper (ii) sulfate (cuso 4) is added to sodium. Your browser does not support the video tag. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. How many grams of zinc(ii) nitrate and sodium sulfide. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. The balanced equation will be calculated along with the. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Enter an equation of an ionic chemical equation and press the balance button.
From www.alamy.com
Precipitation of copper (II) hydroxide. A solution of copper sulphate is forced into a solution Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Sodium hydroxide with copper (ii) sulfate. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. I tried reacting copper sulfate with sodium hydroxide to get copper. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From fineartamerica.com
Copper Hydroxide Precipitate Photograph by Andrew Lambert Photography Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Here, copper (ii) sulfate (cuso 4) is added to sodium. Your browser does not support the video tag. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper(Ii) Sulfate And Sodium Hydroxide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium chloride, sodium hydroxide, or sodium sulfate?. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Your browser does not. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From stock.adobe.com
Interaction of solutions of sodium hydroxide and copper II sulfate. The formation of a blue Copper(Ii) Sulfate And Sodium Hydroxide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. How many grams of zinc(ii) nitrate and sodium sulfide. Your browser does not support the video tag. Sodium chloride, sodium hydroxide, or sodium sulfate?. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate.. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium hydroxide (NaOH)? Cu(NO3)2 Copper(Ii) Sulfate And Sodium Hydroxide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. How many grams of zinc(ii) nitrate and sodium sulfide. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.alamy.com
Copper(II) sulfate and Sodium Hydroxide Pellets in test tube with plug cap. Cosmetic chemicals Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Your browser does not support the video tag. Enter an equation of an ionic chemical equation and press the balance button. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium chloride, sodium hydroxide,. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.chegg.com
Solved Copper(II) sulfate + Sodium hydroxide Blue gelatinous Copper(Ii) Sulfate And Sodium Hydroxide Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Sodium chloride,. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From fphoto.photoshelter.com
science chemistry precipitation reaction cupric hydroxide Fundamental Photographs The Art of Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Here, copper (ii) sulfate (cuso 4) is added to sodium. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Your browser does not support the video tag. The balanced equation will be calculated. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From stock.adobe.com
Double displacement reaction sodium hydroxide and copper sulfate. Types of chemical reactions Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Enter an equation of an ionic chemical equation and press the balance button. How many grams of zinc(ii) nitrate and sodium sulfide. Your browser does not support the video tag. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Use either a net ionic equations (omit the na+), molecular equation (include the copper. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Your browser does not support the video tag. How many grams of zinc(ii) nitrate and sodium sulfide. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium hydroxide with copper (ii) sulfate. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. A typical. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
Copper (II) Sulfate + Sodium Carbonate = (Double Displacement with Precipitate) YouTube Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. Sodium hydroxide with copper (ii) sulfate. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample:. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
Copper(II) Sulfate and Sodium Hydroxide reaction CuSO4 + NaOH DoubleDisplacement Reaction Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Sodium hydroxide with copper (ii) sulfate. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium chloride, sodium hydroxide, or sodium sulfate?. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. Use either a net ionic equations (omit the na+), molecular equation (include. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.doubtnut.com
Copper [II] sulphate solution. Reacts with sodium hydroxide solution to form a precipitate of Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Enter an equation of an ionic chemical equation and press the balance button. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. Here, copper (ii) sulfate (cuso 4) is added to sodium. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Which. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.alamy.com
Copper (II) chloride (CuCl2) solution. When sodium hydroxide is added to a solution of copper Copper(Ii) Sulfate And Sodium Hydroxide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Sodium chloride, sodium hydroxide, or sodium sulfate?. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Sodium hydroxide with copper (ii) sulfate. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.nagwa.com
Question Video Recalling the Color of the Precipitate Forms When Sodium Hydroxide Is Added to Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Here, copper (ii) sulfate (cuso 4) is added to sodium. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. The balanced equation will be calculated along with the. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution.. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From pixels.com
Precipitate Of Copper (ii) Chloride In Sodium Hydroxide Photograph by Martyn F. Chillmaid Copper(Ii) Sulfate And Sodium Hydroxide Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Your browser does not support the video tag. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. The balanced equation will be calculated along with the. Sodium chloride, sodium hydroxide, or. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.slideserve.com
PPT Evidence of Chemical Change Laboratory PowerPoint Presentation, free download ID5875321 Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Sodium chloride, sodium hydroxide, or sodium sulfate?. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium hydroxide with copper (ii) sulfate. I tried reacting copper sulfate with sodium. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.chegg.com
Solved REACTION 5 COPPER SULFATE (CuSO) AND SODIUM HYDROXIDE Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Sodium chloride, sodium hydroxide, or sodium sulfate?. Here, copper (ii) sulfate (cuso 4) is added to sodium. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. The balanced equation will be calculated along with the. Sodium hydroxide with copper (ii) sulfate. Adding 10.0 ml of a dilute solution of zinc. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.alamy.com
Precipitation of copper (II) hydroxide. A solution of copper sulphate is forced into a solution Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Here, copper (ii) sulfate (cuso 4) is added to sodium. How many grams of zinc(ii) nitrate and sodium sulfide. Sodium hydroxide with copper (ii) sulfate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Your browser does not support the video tag. Which solution could be used to precipitate the barium ion,. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
Reaction between copper(II)sulfate and potassium hydroxide YouTube Copper(Ii) Sulfate And Sodium Hydroxide Precipitate I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. The balanced equation will be calculated along with the. How many grams of zinc(ii) nitrate and sodium sulfide. Here, copper (ii) sulfate (cuso 4) is added to sodium. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From brainly.in
What are the products of Copper sulphate + sodium hydroxide? Brainly.in Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Sodium hydroxide with copper (ii) sulfate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium chloride,. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Your browser does not support the video tag. Sodium hydroxide with copper (ii) sulfate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium chloride, sodium hydroxide, or sodium sulfate?. Here, copper (ii) sulfate (cuso 4) is added to sodium. Adding 10.0 ml of a dilute solution of zinc nitrate to 246. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From brainly.in
Copper sulphate reacts with sodium hydroxide to form blue precipitate of copper hydroxide and Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Sodium hydroxide with copper (ii) sulfate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. How many grams of zinc(ii) nitrate and sodium sulfide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The balanced equation will be calculated along with the.. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.storyblocks.com
Interaction Of Solutions Of Sodium Hydroxide Stock Footage SBV315393833 Storyblocks Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Sodium chloride, sodium hydroxide, or sodium sulfate?. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Here, copper (ii) sulfate (cuso 4) is added to sodium.. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
Sodium Hydroxide and Copper Sulfate reaction YouTube Copper(Ii) Sulfate And Sodium Hydroxide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Sodium hydroxide with copper (ii) sulfate. The balanced equation will be calculated along with the. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Enter an equation of an ionic chemical equation and press the balance. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.alamy.com
Zinc Powder, Copper(II) sulfate and Sodium Hydroxide Pellets in test tube with plug cap Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Sodium chloride, sodium hydroxide, or sodium sulfate?. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide with copper (ii) sulfate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Your browser does not support the video tag. How many grams. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
Copper(II) sulfate and sodium hydroxide solutions… Copper(Ii) Sulfate And Sodium Hydroxide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. I tried reacting copper sulfate with sodium hydroxide to get copper. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.slideshare.net
Acids And Bases Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Enter an equation of an ionic chemical equation and press the balance button. Sodium chloride, sodium hydroxide, or sodium sulfate?. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. The balanced equation will be calculated along with the. Which solution could be used to precipitate the barium ion, ba 2+,. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.pinterest.com
Sodium Hydroxide, Stoic, Reactions, Analysis, Development Copper(Ii) Sulfate And Sodium Hydroxide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium chloride, sodium hydroxide, or sodium sulfate?. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Adding. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.meritnation.com
What is formed when CuSO4 reacts with Sodium hydroxide solution Also give it's equation Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium chloride, sodium hydroxide, or sodium sulfate?. Enter. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.alamy.com
Copper hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution containing Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. Your browser does not support the video tag. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.storyblocks.com
Interaction of solutions of sodium hydroxide and copper II sulfate. The formation of a blue Copper(Ii) Sulfate And Sodium Hydroxide Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Adding 10.0 ml of a dilute. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.dreamstime.com
Interaction of Solutions of Sodium Hydroxide and Copper II Sulfate. the Formation of a Blue Copper(Ii) Sulfate And Sodium Hydroxide Precipitate Here, copper (ii) sulfate (cuso 4) is added to sodium. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Your browser does not support the video tag. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following. How many grams of zinc(ii) nitrate. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.chegg.com
Solved copper (II) sulfate + sodium hydroxide → Blue Copper(Ii) Sulfate And Sodium Hydroxide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. Your browser does not support the video tag. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9441 Science Photo Library Copper(Ii) Sulfate And Sodium Hydroxide Precipitate The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. How many grams of zinc(ii) nitrate and sodium sulfide. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: A typical precipitation reaction occurs when an aqueous solution of barium chloride is. Copper(Ii) Sulfate And Sodium Hydroxide Precipitate.