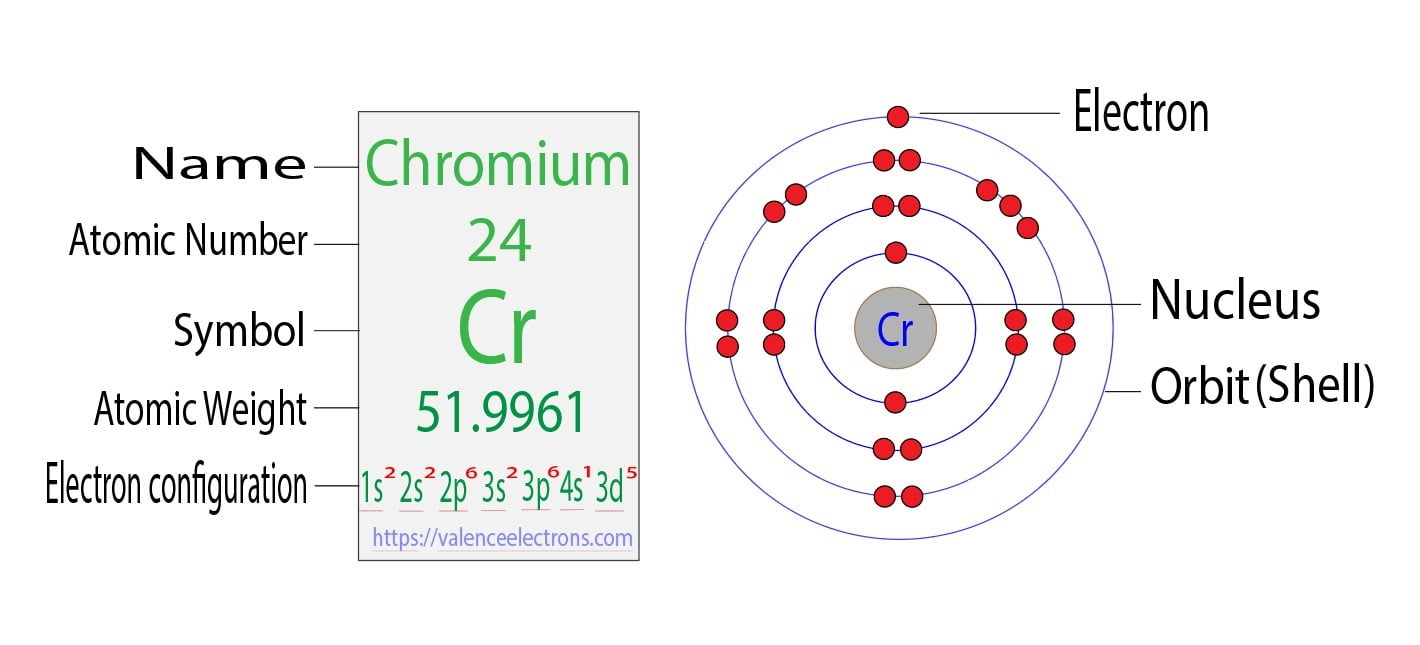
Lead Core Electrons . [xe] 6s 2 4f 14 5d 10 6p 2. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. The total number of neutrons in the nucleus of an. But for most of the transition. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Electron configuration chart of all elements is mentioned in the table below. Lead has 82 protons and electrons in its structure. The shorthand electron configuration (or noble gas configuration) as well as. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The shorthand electron configuration for lead is:
from valenceelectrons.com
The shorthand electron configuration for lead is: The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. But for most of the transition. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. [xe] 6s 2 4f 14 5d 10 6p 2. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. Lead has 82 protons and electrons in its structure. The total number of neutrons in the nucleus of an. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. Electron configuration chart of all elements is mentioned in the table below.
Electron Configuration for Lead and Lead ions(Pb2+, Pb4+)
Lead Core Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The shorthand electron configuration for lead is: Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. The shorthand electron configuration (or noble gas configuration) as well as. The total number of neutrons in the nucleus of an. [xe] 6s 2 4f 14 5d 10 6p 2. Lead has 82 protons and electrons in its structure. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Electron configuration chart of all elements is mentioned in the table below. But for most of the transition. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise.
From www.alamy.com
Lead atom, with mass and energy levels. Vector illustration Stock Vector Image & Art Alamy Lead Core Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The total number of neutrons in the nucleus of an. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. The shorthand electron configuration (or noble gas configuration) as well as. Electron. Lead Core Electrons.
From periodictable.me
Valency of Lead Archives Dynamic Periodic Table of Elements and Chemistry Lead Core Electrons Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The total number of neutrons in the nucleus of an. The shorthand electron configuration for lead is: The shorthand electron configuration (or noble gas configuration) as well as. [xe] 6s 2 4f 14 5d 10 6p 2. The electrons. Lead Core Electrons.
From awesomehome.co
Lead Periodic Table Electrons Awesome Home Lead Core Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the. Lead Core Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Core Electrons Lead has 82 protons and electrons in its structure. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. The total number of neutrons in the nucleus of an. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2. Lead Core Electrons.
From www.dreamstime.com
Lead stock illustration. Illustration of atomic, symbol 175796461 Lead Core Electrons Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. Lead has 82 protons and electrons in its structure. [xe] 6s 2 4f 14 5d 10 6p 2. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p. Lead Core Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Core Electrons Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The total number of neutrons in the nucleus of an. But for most of the transition. Electron configuration chart of all elements. Lead Core Electrons.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 Resource Book Lead Core Electrons The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. The total number of neutrons in the nucleus of an. The. Lead Core Electrons.
From exovwrlic.blob.core.windows.net
Pb Lead Valence Electrons at Eve Dehart blog Lead Core Electrons The total number of neutrons in the nucleus of an. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Electron configuration chart of all elements is mentioned in the table below. Lead has 82 protons and electrons in its structure. Learn the basics of electron configurations before attempting. Lead Core Electrons.
From www.alamy.com
Atom symbol and electron of lead illustration Stock Vector Image & Art Alamy Lead Core Electrons The shorthand electron configuration for lead is: Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The shorthand electron configuration (or noble gas configuration) as well as. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The total number of. Lead Core Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Lead has 82 protons and electrons in its structure. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Lead Core Electrons.
From www.slideserve.com
PPT Arrangement of Electrons in Atoms PowerPoint Presentation, free download ID6299288 Lead Core Electrons Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner. Lead Core Electrons.
From www.slideserve.com
PPT Key Terms Chapter 7 PowerPoint Presentation, free download ID2979424 Lead Core Electrons [xe] 6s 2 4f 14 5d 10 6p 2. The shorthand electron configuration (or noble gas configuration) as well as. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Electron configuration. Lead Core Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Core Electrons [xe] 6s 2 4f 14 5d 10 6p 2. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. The total. Lead Core Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s. Lead Core Electrons.
From exoroknts.blob.core.windows.net
Lead Outermost Electrons at Donnie Kell blog Lead Core Electrons Electron configuration chart of all elements is mentioned in the table below. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. But for most of the transition. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The lead electron configuration,. Lead Core Electrons.
From www.youtube.com
CHEMISTRY 101 Valence and core electrons YouTube Lead Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. [xe] 6s 2 4f 14 5d 10 6p 2. The shorthand electron configuration for lead is: Electron configuration chart of all elements is mentioned in the table below. Lead has 82 protons and electrons in its structure. But for. Lead Core Electrons.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Lead Core Electrons The shorthand electron configuration for lead is: But for most of the transition. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. [xe] 6s 2 4f 14 5d 10. Lead Core Electrons.
From eightfoldlearning.com
Effective Nuclear Charge Eightfold Lead Core Electrons The total number of neutrons in the nucleus of an. The shorthand electron configuration (or noble gas configuration) as well as. The shorthand electron configuration for lead is: The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Electron configuration chart of all elements is mentioned in the table. Lead Core Electrons.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Organic chemistry Lead Core Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The shorthand electron configuration for lead is: Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons,. Lead Core Electrons.
From exovwrlic.blob.core.windows.net
Pb Lead Valence Electrons at Eve Dehart blog Lead Core Electrons Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s. Lead Core Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead Core Electrons The shorthand electron configuration (or noble gas configuration) as well as. The shorthand electron configuration for lead is: Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. [xe] 6s 2 4f 14 5d 10 6p 2. Lead has 82 protons and electrons in its structure. The electrons occupying. Lead Core Electrons.
From periodictable.me
How Many Valence Electron Configuration For Lead (Pb) Lead Core Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. [xe] 6s 2 4f 14 5d 10 6p 2. Lead has 82 protons and electrons in its structure. The total number of neutrons in the nucleus of an. Lead is a chemical element with atomic number 82 which means there are 82 protons. Lead Core Electrons.
From material-properties.org
Lead Protons Neutrons Electrons Electron Configuration Lead Core Electrons [xe] 6s 2 4f 14 5d 10 6p 2. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. Lead has. Lead Core Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead Core Electrons The shorthand electron configuration for lead is: Lead has 82 protons and electrons in its structure. Electron configuration chart of all elements is mentioned in the table below. But for most of the transition. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. [xe] 6s 2 4f 14 5d 10 6p 2.. Lead Core Electrons.
From www.youtube.com
CHEMISTRY 101 Writing an Electron Configuration for Lead Using the Periodic Table YouTube Lead Core Electrons The shorthand electron configuration for lead is: Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Lead has 82 protons and electrons in its structure. But for most of the transition. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic.. Lead Core Electrons.
From www.alamy.com
Atom symbol electron lead illustration hires stock photography and images Alamy Lead Core Electrons But for most of the transition. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Lead has 82 protons and electrons in its structure. [xe] 6s 2 4f 14 5d 10 6p 2. Electron configuration chart of all elements is mentioned in the table below. Lead is a. Lead Core Electrons.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Table of the Elements Lead Core Electrons Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. But for most of the transition. Electron configuration chart of all elements is mentioned in the table below. The total number of neutrons in the nucleus of an. Learn the basics of electron configurations before attempting to write out. Lead Core Electrons.
From valenceelectrons.com
Electron Configuration for Lead and Lead ions(Pb2+, Pb4+) Lead Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. But for most of the transition. The shorthand electron configuration (or noble gas configuration) as well as. Lead has 82 protons and electrons in its structure. The shorthand electron configuration for lead is: The total number of neutrons in. Lead Core Electrons.
From www.slideserve.com
PPT Chapter 7 “Ionic and Metallic Bonding” PowerPoint Presentation ID6732018 Lead Core Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. But for most of the transition. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration. Lead Core Electrons.
From chio-pixie.blogspot.com
Valence Electrons Examples Counting Valence Electrons For Main Group Elements Video Khan Lead Core Electrons But for most of the transition. Electron configuration chart of all elements is mentioned in the table below. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p. Lead Core Electrons.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Bismuth vs Lead Compare Properties, Structure, Facts Lead Core Electrons Lead has 82 protons and electrons in its structure. But for most of the transition. The shorthand electron configuration for lead is: Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements. Lead Core Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Core Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. The shorthand electron configuration for lead is: The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of. Lead Core Electrons.
From periodictable.me
Lead Valence Electrons Lead Valency (Pb) with Dot Diagram Lead Core Electrons The total number of neutrons in the nucleus of an. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. [xe] 6s 2 4f 14 5d 10 6p 2. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Lead has 82. Lead Core Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Core Electrons Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2. Lead Core Electrons.
From www.slideserve.com
PPT Core Electrons PowerPoint Presentation, free download ID6788046 Lead Core Electrons [xe] 6s 2 4f 14 5d 10 6p 2. The shorthand electron configuration (or noble gas configuration) as well as. But for most of the transition. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2. Lead Core Electrons.