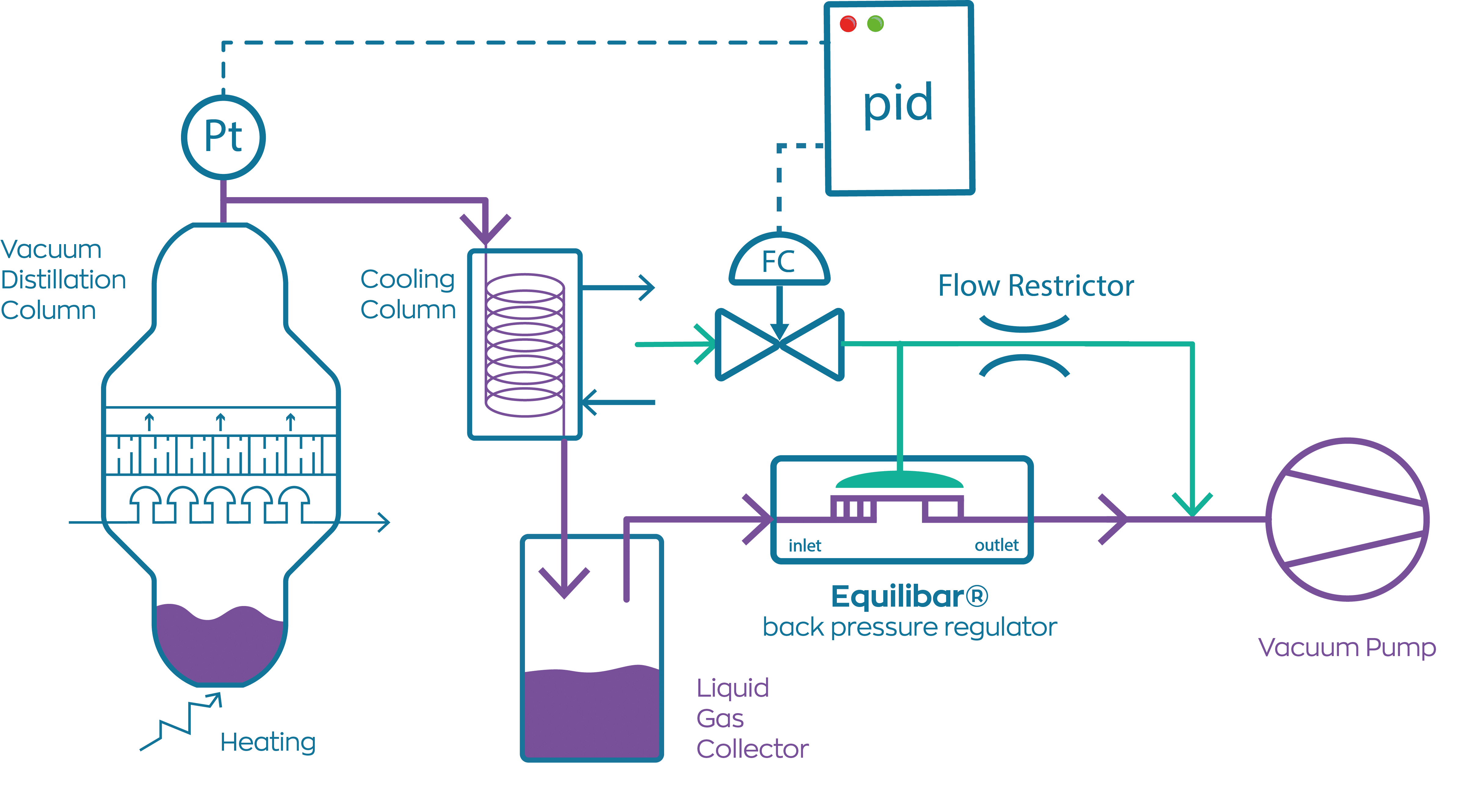
What Is Vacuum Distillation In Chemistry . A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced. Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. This is particularly useful for distilling products of. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. A fraction distillation can also be used.
from www.pressurecontrolsolutions.com
a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Boiling commences when the vapor pressure of a liquid or solution equals the external. A fraction distillation can also be used. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). This is particularly useful for distilling products of. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced.
Vacuum Distillation issues? Call Pressure Control Solutions!
What Is Vacuum Distillation In Chemistry Boiling commences when the vapor pressure of a liquid or solution equals the external. This is particularly useful for distilling products of. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced. Boiling commences when the vapor pressure of a liquid or solution equals the external. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. A fraction distillation can also be used. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48).
From www.youtube.com
Vacuum Distillation YouTube What Is Vacuum Distillation In Chemistry This is particularly useful for distilling products of. Boiling commences when the vapor pressure of a liquid or solution equals the external. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric. What Is Vacuum Distillation In Chemistry.
From chem.libretexts.org
5.3D StepbyStep Procedures for Fractional Distillation Chemistry What Is Vacuum Distillation In Chemistry A fraction distillation can also be used. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple. What Is Vacuum Distillation In Chemistry.
From www.chemistry-online.com
Vacuum distillation Chemistry Online What Is Vacuum Distillation In Chemistry vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. A fraction distillation can also be used. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced. A vacuum distillation apparatus is shown in figure. What Is Vacuum Distillation In Chemistry.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] What Is Vacuum Distillation In Chemistry When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. This is particularly useful for distilling products of. Boiling commences when the vapor pressure of a liquid or solution equals the external. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. A vacuum distillation apparatus. What Is Vacuum Distillation In Chemistry.
From www.periodni.com
Download distillation.png image from What Is Vacuum Distillation In Chemistry vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is performed. What Is Vacuum Distillation In Chemistry.
From www.thoughtco.com
What Is Distillation? Principles and Uses What Is Vacuum Distillation In Chemistry vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A fraction distillation can also be used.. What Is Vacuum Distillation In Chemistry.
From www.researchgate.net
Fig. Atmosphere and Vacuum Distillation Download Scientific Diagram What Is Vacuum Distillation In Chemistry This is particularly useful for distilling products of. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A fraction distillation. What Is Vacuum Distillation In Chemistry.
From www.researchgate.net
The schematic for the vacuum distillation process. Download What Is Vacuum Distillation In Chemistry When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. This is particularly useful for distilling products of. Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order. What Is Vacuum Distillation In Chemistry.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review What Is Vacuum Distillation In Chemistry It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. a vacuum distillation is performed. What Is Vacuum Distillation In Chemistry.
From foodtechnotes.com
Distillation Principle and Types Food Tech Notes What Is Vacuum Distillation In Chemistry A fraction distillation can also be used. Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. vacuum distillation is a distillation carried out. What Is Vacuum Distillation In Chemistry.
From www.worksheetsplanet.com
What is Distillation Definition of Distillation What Is Vacuum Distillation In Chemistry a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. A fraction distillation can also be used. Boiling commences when the vapor pressure of a liquid or solution equals the external. vacuum distillation is a process used by. What Is Vacuum Distillation In Chemistry.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form What Is Vacuum Distillation In Chemistry It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced. Boiling commences when the vapor pressure of a liquid or solution equals the external. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. A. What Is Vacuum Distillation In Chemistry.
From chemicaltweak.com
When Vacuum Distillation Is Selected Application And Uses Of VDU What Is Vacuum Distillation In Chemistry a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A fraction distillation can also. What Is Vacuum Distillation In Chemistry.
From www.etsy.com
Distillation Apparatus Diagram Poster Printable Lab Tools Etsy What Is Vacuum Distillation In Chemistry vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent.. What Is Vacuum Distillation In Chemistry.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts What Is Vacuum Distillation In Chemistry a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. This is particularly useful for distilling products of. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional. What Is Vacuum Distillation In Chemistry.
From mavink.com
Vacuum Distillation Set Up What Is Vacuum Distillation In Chemistry Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. This. What Is Vacuum Distillation In Chemistry.
From www.processingmagazine.com
Controlling chemical vacuum processes with direct sealing diaphragm What Is Vacuum Distillation In Chemistry When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. A fraction distillation can also be used. Boiling commences when the vapor pressure of a liquid or solution equals the external. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using. What Is Vacuum Distillation In Chemistry.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram What Is Vacuum Distillation In Chemistry This is particularly useful for distilling products of. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. a vacuum distillation is used when the boiling point of the compound (or the solvent) is. What Is Vacuum Distillation In Chemistry.
From narodnatribuna.info
Simple Distillation Distillation Chemistry Lessons Teaching Chemistry What Is Vacuum Distillation In Chemistry Boiling commences when the vapor pressure of a liquid or solution equals the external. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. This is particularly useful for distilling products of. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. a vacuum distillation is performed by applying a. What Is Vacuum Distillation In Chemistry.
From chemicalengineeringworld.com
Types of Distillation Chemical Engineering World What Is Vacuum Distillation In Chemistry Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. vacuum distillation is a process used by. What Is Vacuum Distillation In Chemistry.
From mavink.com
Vacuum Distillation Set Up What Is Vacuum Distillation In Chemistry A fraction distillation can also be used. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order. What Is Vacuum Distillation In Chemistry.
From pharmaguides.in
2.0 Distillation Chemistry What Is Distillation? What Is Vacuum Distillation In Chemistry Boiling commences when the vapor pressure of a liquid or solution equals the external. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. This is particularly useful for distilling products of. A fraction distillation can also be used. When the pressure is lowered inside the apparatus, solutions boil at. What Is Vacuum Distillation In Chemistry.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] What Is Vacuum Distillation In Chemistry When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o. What Is Vacuum Distillation In Chemistry.
From www.britannica.com
distillation summary Britannica What Is Vacuum Distillation In Chemistry When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. A fraction distillation can also be used. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. a vacuum distillation is performed by applying. What Is Vacuum Distillation In Chemistry.
From www.shalom-education.com
Simple and Fractional Distillation KS3 Chemistry Revision What Is Vacuum Distillation In Chemistry vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. A. What Is Vacuum Distillation In Chemistry.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form What Is Vacuum Distillation In Chemistry This is particularly useful for distilling products of. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order. What Is Vacuum Distillation In Chemistry.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry What Is Vacuum Distillation In Chemistry a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so. What Is Vacuum Distillation In Chemistry.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts What Is Vacuum Distillation In Chemistry Boiling commences when the vapor pressure of a liquid or solution equals the external. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this. What Is Vacuum Distillation In Chemistry.
From gioywzykw.blob.core.windows.net
Vacuum Distillation Definition Chemistry at Cynthia Stone blog What Is Vacuum Distillation In Chemistry vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. A fraction distillation can also be used. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order. What Is Vacuum Distillation In Chemistry.
From www.vrogue.co
Types Of Distillation Definition Process Uses Example vrogue.co What Is Vacuum Distillation In Chemistry vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. This is particularly useful for distilling products of. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric. What Is Vacuum Distillation In Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Distillation (simple What Is Vacuum Distillation In Chemistry a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A fraction distillation can also be used. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or. What Is Vacuum Distillation In Chemistry.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry What Is Vacuum Distillation In Chemistry A fraction distillation can also be used. vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. vacuum distillation is a process used. What Is Vacuum Distillation In Chemistry.
From www.researchgate.net
Schematic Process Flow Diagram for Vacuum Distillation Download What Is Vacuum Distillation In Chemistry vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). This is. What Is Vacuum Distillation In Chemistry.
From easywayscience78.blogspot.com
Distillation Easy way to learn science What Is Vacuum Distillation In Chemistry Boiling commences when the vapor pressure of a liquid or solution equals the external. This is particularly useful for distilling products of. vacuum distillation is a process used by industries that deal with liquids that may contain various compounds, such as. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too. What Is Vacuum Distillation In Chemistry.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! What Is Vacuum Distillation In Chemistry vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A fraction distillation can also be used. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup.. What Is Vacuum Distillation In Chemistry.