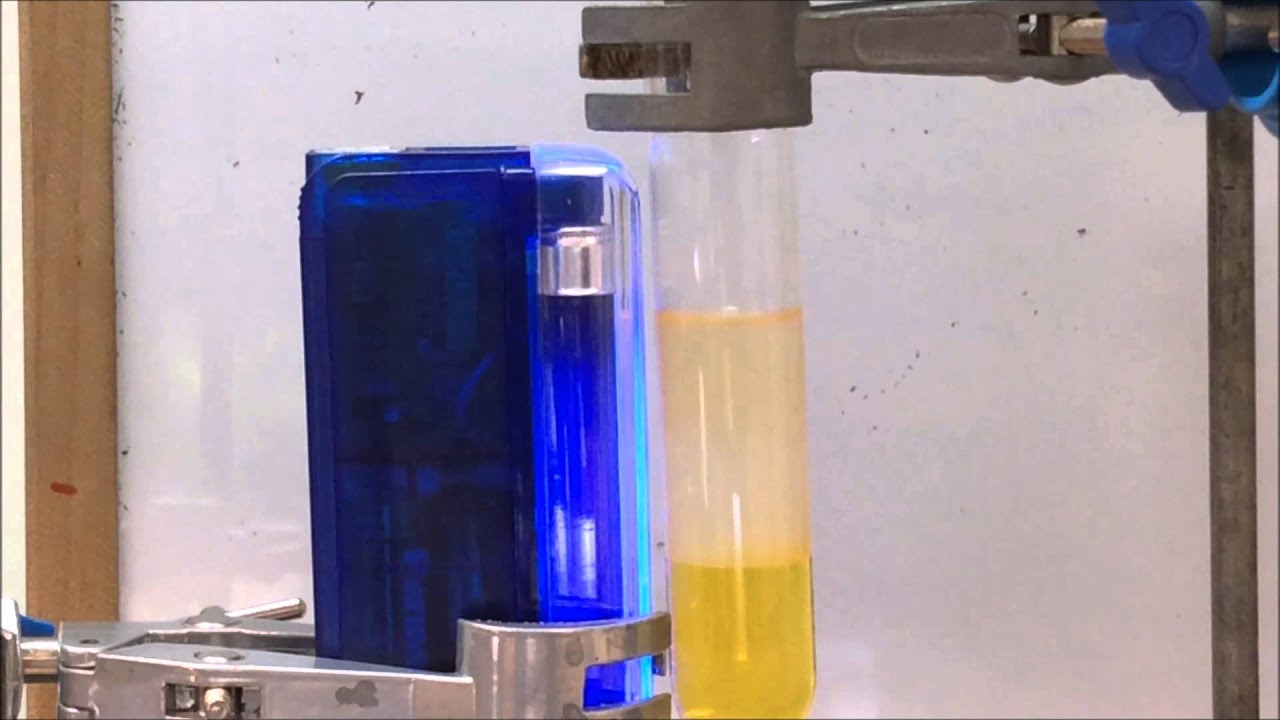
Bromine Water Added To Hexene . testing for alkanes and alkenes. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction is as follows: the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; But when bromine water is added to an alkene, the. 11k views 3 years ago. the electrophilic addition of bromine to ethene. addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. there is no change when bromine water is mixed with an alkane; The bromine water becomes colourless when it is mixed with an alkene
from www.youtube.com
The reaction is as follows: But when bromine water is added to an alkene, the. addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. there is no change when bromine water is mixed with an alkane; The bromine water becomes colourless when it is mixed with an alkene testing for alkanes and alkenes. the electrophilic addition of bromine to ethene. when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; 11k views 3 years ago.
Bromination of Hexane in the Presence of UV Light YouTube
Bromine Water Added To Hexene testing for alkanes and alkenes. when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; testing for alkanes and alkenes. the electrophilic addition of bromine to ethene. The bromine water becomes colourless when it is mixed with an alkene But when bromine water is added to an alkene, the. The reaction is as follows: Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. there is no change when bromine water is mixed with an alkane; the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. 11k views 3 years ago.
From mungfali.com
Hexene And Bromine Reaction Bromine Water Added To Hexene 11k views 3 years ago. the electrophilic addition of bromine to ethene. The bromine water becomes colourless when it is mixed with an alkene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction is as follows: there is no change when bromine water is. Bromine Water Added To Hexene.
From quintensrtate.blogspot.com
Colour of Bromine Water QuintensrTate Bromine Water Added To Hexene there is no change when bromine water is mixed with an alkane; addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. The bromine water becomes colourless when it is mixed with an. Bromine Water Added To Hexene.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine Water Added To Hexene when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; the electrophilic addition of bromine to ethene. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. But when. Bromine Water Added To Hexene.
From www.echemi.com
Bromination of hexene in presence of UV light or heat ECHEMI Bromine Water Added To Hexene the electrophilic addition of bromine to ethene. when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; The reaction is as follows: But when bromine water is added to an alkene, the. addition of water to alkenes. Bromine Water Added To Hexene.
From www.alamy.com
Alkane decolourisation of bromine water. Image 1 of 2. Start of the Bromine Water Added To Hexene the electrophilic addition of bromine to ethene. addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in. Bromine Water Added To Hexene.
From www.slideserve.com
PPT alkenes PowerPoint Presentation, free download ID149452 Bromine Water Added To Hexene 11k views 3 years ago. the electrophilic addition of bromine to ethene. there is no change when bromine water is mixed with an alkane; The bromine water becomes colourless when it is mixed with an alkene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. . Bromine Water Added To Hexene.
From www.youtube.com
Bromine Water + Sodium Chloride YouTube Bromine Water Added To Hexene the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. The reaction is as follows: when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; Alkenes react in the cold. Bromine Water Added To Hexene.
From joelgordon.photoshelter.com
Reaction of bromine with an unsaturated hydrocarbon Joel Gordon Bromine Water Added To Hexene when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. addition of water to alkenes (hydration of alkenes) an. Bromine Water Added To Hexene.
From www.lelandjansen.com
Chemistry Labs — Leland Jansen Bromine Water Added To Hexene when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; The reaction is as follows: the electrophilic addition of bromine to ethene. there is no change when bromine water is mixed with an alkane; Alkenes react in. Bromine Water Added To Hexene.
From www.echemi.com
Reactions between bromine and hexene ECHEMI Bromine Water Added To Hexene there is no change when bromine water is mixed with an alkane; testing for alkanes and alkenes. But when bromine water is added to an alkene, the. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. the reaction between hexene, bromine, and water is an. Bromine Water Added To Hexene.
From www.numerade.com
SOLVED Using condensed structure, write the balanced chemical equation Bromine Water Added To Hexene testing for alkanes and alkenes. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. the electrophilic addition of bromine to ethene. when bromine water is added. Bromine Water Added To Hexene.
From fineartamerica.com
Bromine Test For Alkene Photograph by Andrew Lambert Photography Bromine Water Added To Hexene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; addition of water to alkenes (hydration of. Bromine Water Added To Hexene.
From www.i-ciencias.com
[Resuelta] organicchemistry Sustitución electrofílica de Bromine Water Added To Hexene the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. But when bromine water is added to an alkene, the. addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an. Bromine Water Added To Hexene.
From www.alamy.com
Bromine water test for alkenes. When an alkene is added to a solution Bromine Water Added To Hexene the electrophilic addition of bromine to ethene. addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in. Bromine Water Added To Hexene.
From www.youtube.com
Identifying Alkenes with Bromine Water YouTube Bromine Water Added To Hexene 11k views 3 years ago. testing for alkanes and alkenes. But when bromine water is added to an alkene, the. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. there is no change when bromine water is mixed with an alkane; when bromine water is added to. Bromine Water Added To Hexene.
From www.youtube.com
Organic Chemistry Testing for alkenes using bromine water YouTube Bromine Water Added To Hexene when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; 11k views 3 years ago. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. there is no change. Bromine Water Added To Hexene.
From saniyahghopdalton.blogspot.com
Reaction of Cyclohexene With Bromine Bromine Water Added To Hexene 11k views 3 years ago. The bromine water becomes colourless when it is mixed with an alkene the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. there is no change when bromine water is mixed with an alkane; the electrophilic addition of bromine to ethene. testing for. Bromine Water Added To Hexene.
From www.youtube.com
Testing alkane & alkene with bromine water YouTube Bromine Water Added To Hexene addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. the electrophilic addition of bromine to ethene. But when bromine water is added to an alkene, the. the reaction between hexene, bromine,. Bromine Water Added To Hexene.
From www.alamy.com
Alkane decolourisation of bromine water. Image 2 of 2. End result of Bromine Water Added To Hexene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine water becomes colourless when it is mixed with an alkene when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. Bromine Water Added To Hexene.
From www.youtube.com
Bromine Water Test Explained// HSC Chemistry YouTube Bromine Water Added To Hexene the electrophilic addition of bromine to ethene. 11k views 3 years ago. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine. Bromine Water Added To Hexene.
From mungfali.com
Hexene And Bromine Reaction Bromine Water Added To Hexene 11k views 3 years ago. testing for alkanes and alkenes. when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a. Bromine Water Added To Hexene.
From www.alamy.com
Alkane/alkene in bromine water. Test tubes containing the alkenes Bromine Water Added To Hexene when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; there is no change when bromine water is mixed with an alkane; 11k views 3 years ago. The bromine water becomes colourless when it is mixed with an. Bromine Water Added To Hexene.
From www.chegg.com
Solved Bromine water is added to 1hexene > major product Bromine Water Added To Hexene 11k views 3 years ago. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; addition of water to. Bromine Water Added To Hexene.
From www.youtube.com
Bromine Water Experiment YouTube Bromine Water Added To Hexene when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act. Bromine Water Added To Hexene.
From scienceready.com.au
Bromine Water Test Science Ready Bromine Water Added To Hexene But when bromine water is added to an alkene, the. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction is as follows: when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds. Bromine Water Added To Hexene.
From www.numerade.com
SOLVED QUESTION 4 Two test tubes A and B contain hex1ene and hexane Bromine Water Added To Hexene the electrophilic addition of bromine to ethene. testing for alkanes and alkenes. The bromine water becomes colourless when it is mixed with an alkene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. the reaction between hexene, bromine, and water is an addition reaction resulting. Bromine Water Added To Hexene.
From proper-cooking.info
Bromine Gas Equation Bromine Water Added To Hexene 11k views 3 years ago. there is no change when bromine water is mixed with an alkane; Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. The bromine. Bromine Water Added To Hexene.
From www.coursehero.com
[Solved] Bromine water (golden yellow solution) and the purple Bromine Water Added To Hexene when bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the bromine remains in solution; Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. addition of water to alkenes (hydration of. Bromine Water Added To Hexene.
From www.sciencephoto.com
Alkane decolourisation of bromine water Stock Image A500/0723 Bromine Water Added To Hexene The bromine water becomes colourless when it is mixed with an alkene the electrophilic addition of bromine to ethene. The reaction is as follows: testing for alkanes and alkenes. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. 11k views 3 years ago. when bromine water is. Bromine Water Added To Hexene.
From www.gauthmath.com
The test tube contains bromine, water, and hexane. Which layer best Bromine Water Added To Hexene addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. 11k views 3 years ago. testing for alkanes and alkenes. there is no change when bromine water is mixed with an alkane;. Bromine Water Added To Hexene.
From jessica-has-collier.blogspot.com
Cyclohexane Reaction With Bromine JessicahasCollier Bromine Water Added To Hexene testing for alkanes and alkenes. 11k views 3 years ago. The bromine water becomes colourless when it is mixed with an alkene the electrophilic addition of bromine to ethene. The reaction is as follows: there is no change when bromine water is mixed with an alkane; the reaction between hexene, bromine, and water is an addition. Bromine Water Added To Hexene.
From www.youtube.com
Bromination of Hexane in the Presence of UV Light YouTube Bromine Water Added To Hexene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine water becomes colourless when it is mixed with an alkene But when bromine water is added to an alkene, the. the electrophilic addition of bromine to ethene. addition of water to alkenes (hydration of alkenes). Bromine Water Added To Hexene.
From pixels.com
Alkene Bromine Test Photograph by Andrew Lambert Photography Pixels Bromine Water Added To Hexene addition of water to alkenes (hydration of alkenes) an alkene does not react with pure water, since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. The bromine water becomes colourless. Bromine Water Added To Hexene.
From alaynameowzavala.blogspot.com
Cyclohexane Reaction With Bromine Bromine Water Added To Hexene the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. The reaction is as follows: But when bromine water is added to an alkene, the. testing for alkanes and alkenes. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like. Bromine Water Added To Hexene.
From www.chemedx.org
Creating a Time Lapse Video for the Bromination of Hexane in the Bromine Water Added To Hexene the reaction between hexene, bromine, and water is an addition reaction resulting in the formation of a bromohydrin. The reaction is as follows: The bromine water becomes colourless when it is mixed with an alkene testing for alkanes and alkenes. But when bromine water is added to an alkene, the. when bromine water is added to an. Bromine Water Added To Hexene.