Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry . like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. finally, sublimation is the process in which a solid is change to a gas. Explain the relation between phase transition temperatures and intermolecular attractive forces. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Define phase transitions and phase transition temperatures. Its complement, deposition , is defined as a conversion. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. what is the sublimation process? The formation of frost is an example of. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. The reverse process of sublimation is called deposition, a process. Sublimation, including melting, freezing, and evaporation, is a form of.
from www.numerade.com
Sublimation, including melting, freezing, and evaporation, is a form of. what is the sublimation process? The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. finally, sublimation is the process in which a solid is change to a gas. Define phase transitions and phase transition temperatures. The formation of frost is an example of. Explain the relation between phase transition temperatures and intermolecular attractive forces. The reverse process of sublimation is called deposition, a process. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas.
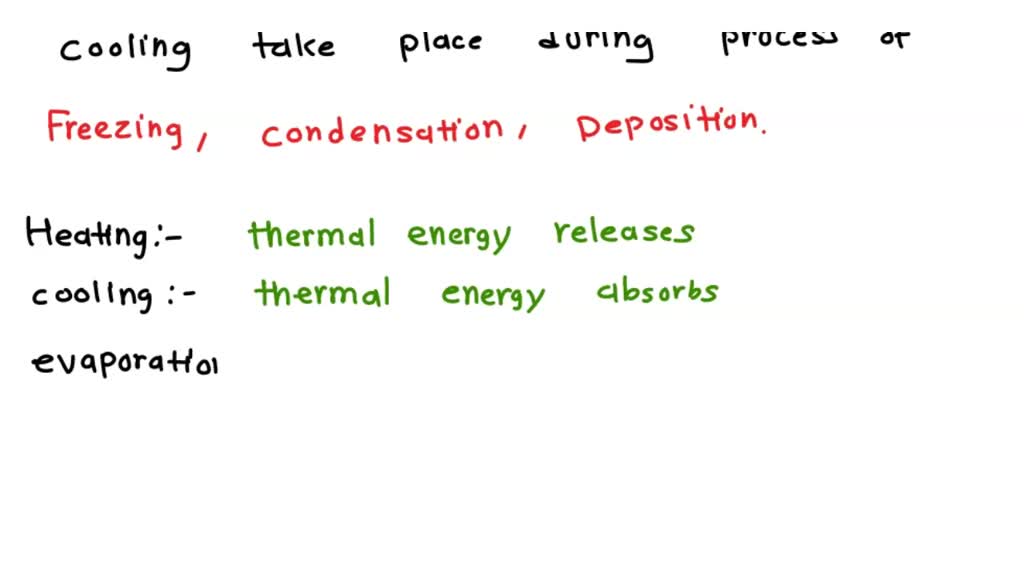
SOLVED Determine whether heating or cooling takes place during each
Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. what is the sublimation process? Explain the relation between phase transition temperatures and intermolecular attractive forces. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. Its complement, deposition , is defined as a conversion. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. finally, sublimation is the process in which a solid is change to a gas. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. The formation of frost is an example of. Sublimation, including melting, freezing, and evaporation, is a form of. like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. The reverse process of sublimation is called deposition, a process. Define phase transitions and phase transition temperatures.
From www.teachoo.com
For any substance, why does the temperature remain constant during the Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry what is the sublimation process? Sublimation, including melting, freezing, and evaporation, is a form of. Define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and intermolecular attractive forces. The formation of frost is an example of. The reverse process of sublimation is called deposition, a process. like vaporization, the process of sublimation requires. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.teachoo.com
What is Sublimation? 5+ Examples with Diagram Teachoo Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry The formation of frost is an example of. what is the sublimation process? The reverse process of sublimation is called deposition, a process. Explain the relation between phase transition temperatures and intermolecular attractive forces. Sublimation, including melting, freezing, and evaporation, is a form of. Its complement, deposition , is defined as a conversion. Define phase transitions and phase transition. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From judith-kconner.blogspot.com
Explain the Difference Between Evaporation and Sublimation Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Define phase transitions and phase transition temperatures. Sublimation, including melting, freezing, and evaporation, is a form of. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. The formation of frost is an example of. what is the sublimation process? Explain the relation between phase transition temperatures and intermolecular attractive forces. The reverse process. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry The formation of frost is an example of. The reverse process of sublimation is called deposition, a process. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. what is the sublimation process? Its complement, deposition , is defined as a conversion. finally, sublimation is the process in which a. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From studiousguy.com
7 Sublimation Examples in Daily Life StudiousGuy Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. The formation of frost is an example of. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Its complement, deposition , is. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From apollo.nvu.vsc.edu
Latent Heat of evaporation, fusion, and freezing Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. finally, sublimation is the process in which a solid is change to a gas. like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. Define phase transitions and phase transition temperatures. Solid co 2, also. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.youtube.com
Change of State Evaporation Condensation Melting Freezing Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. Its complement, deposition , is defined as a conversion. The reverse process of sublimation is called deposition, a process. Define phase transitions and phase transition temperatures. The. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry finally, sublimation is the process in which a solid is change to a gas. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. The formation of frost. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From education-portal.com
Phase Change Evaporation, Condensation, Freezing, Melting, Sublimation Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry The reverse process of sublimation is called deposition, a process. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. Its complement, deposition , is defined as a conversion. what is the sublimation process? Sublimation, including melting, freezing, and evaporation, is a form of. Solid. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From data.allenai.org
changes of state (lesson 0771) TQA explorer Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. Its complement, deposition , is defined as a conversion. The reverse process of sublimation is called deposition, a process. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Explain the relation. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From blogdejuanmateacher.blogspot.com
El blog de juanmateacher 6º CHANGES OF STATE OF MATTER Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry what is the sublimation process? The reverse process of sublimation is called deposition, a process. finally, sublimation is the process in which a solid is change to a gas. Sublimation, including melting, freezing, and evaporation, is a form of. Its complement, deposition , is defined as a conversion. The formation of frost is an example of. Dry ice. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From judith-kconner.blogspot.com
Explain the Difference Between Evaporation and Sublimation Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. The reverse process of sublimation is called deposition, a process. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. finally, sublimation. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.researchgate.net
Schematic view of what happens in a fusionevaporation reaction. In Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry finally, sublimation is the process in which a solid is change to a gas. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Define phase transitions and. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From data.allenai.org
changes of state (lesson 0771) TQA explorer Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Define phase transitions and phase transition temperatures. Its complement, deposition , is defined as a conversion. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. Explain the relation between phase transition temperatures and intermolecular attractive forces. The formation of frost is an example of. Dry. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.numerade.com
SOLVED Determine whether heating or cooling takes place during each Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. finally, sublimation. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From app.emaze.com
on emaze Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. what is the sublimation. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.expii.com
Sublimation — Definition & Overview Expii Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. finally, sublimation is the process in which a solid is change to a gas. Explain the relation between phase transition temperatures and intermolecular attractive forces. Sublimation, including melting, freezing, and evaporation, is a form of. Its complement, deposition , is defined. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From stock.adobe.com
Physical states of matter.Solid, liquid and gas.Melting, freezing Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Its complement, deposition , is defined as a conversion. Define phase transitions and phase transition temperatures. The reverse process of sublimation is called deposition, a process. Sublimation, including melting, freezing, and evaporation, is a form of. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state.. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.youtube.com
Changing states of matter 🔁 Melting, Freezing, Evaporation Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Define phase transitions and phase transition temperatures. Sublimation, including melting, freezing, and evaporation, is a form of. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid state. finally, sublimation is the process in which a solid is change to a gas. Explain the relation between. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.vecteezy.com
Diagram showing Evaporation Separating Mixtures 3176406 Vector Art at Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. The reverse process of sublimation is called deposition, a process. The formation of frost is an example of. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Define phase transitions. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From ar.inspiredpencil.com
Evaporation Chemistry Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry The formation of frost is an example of. like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. The reverse process of sublimation is called deposition, a process. the reverse of sublimation is called deposition, a process in. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From chemistnotes.com
Sublimation Definition/Meaning Chemistry Notes Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry what is the sublimation process? Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. The formation of frost is an example of. The reverse process of sublimation is called deposition, a process. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From judith-kconner.blogspot.com
Explain the Difference Between Evaporation and Sublimation Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry what is the sublimation process? Sublimation, including melting, freezing, and evaporation, is a form of. Its complement, deposition , is defined as a conversion. like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. The reverse process of. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From stacklima.com
Qu’estce que la vaporisation ? StackLima Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Explain the relation between phase transition temperatures and intermolecular attractive forces. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. The reverse process of sublimation is called deposition, a process. . Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Its complement, deposition , is defined as a conversion. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. The reverse process of sublimation is called deposition, a process. Explain the relation. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.youtube.com
Science 9 Chapter 2 Evaporation sublimation, Chromatography Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Define phase transitions and phase transition temperatures. The reverse process of sublimation is called deposition, a process. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Its complement, deposition , is. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.researchgate.net
1 Schematic view of the fusionevaporation reactions process. The Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Explain the relation between phase transition temperatures and intermolecular attractive forces. Sublimation, including melting, freezing, and evaporation, is a form of. Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. finally, sublimation is the process in which a solid is change to a gas. The. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From kaia-has-orr.blogspot.com
Explain the Difference Between Evaporation and Sublimation KaiahasOrr Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. Dry ice undergoes sublimation to change from solid carbon dioxide directly into carbon dioxide gas. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. The reverse process of sublimation is called deposition, a process. Explain. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.researchgate.net
a) Compiled experimental ER cross sections of fusionevaporation Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. finally, sublimation is the process in which a solid is change to a gas. Explain the relation between phase transition temperatures and intermolecular attractive forces. like vaporization, the process of sublimation requires an input of. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From app.emaze.com
Science Molecules on emaze Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry The formation of frost is an example of. finally, sublimation is the process in which a solid is change to a gas. what is the sublimation process? Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. like vaporization, the process of sublimation requires. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.youtube.com
What is sublimation fusion and why evaporation causes cooling class 9th Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry what is the sublimation process? Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. the reverse of sublimation is called deposition, a process in which gaseous substances condense. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. Sublimation, including melting, freezing, and evaporation, is a form of. The formation of frost is an example of. Explain the relation between phase transition temperatures and intermolecular attractive forces. the reverse of sublimation is called deposition,. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From childhealthpolicy.vumc.org
🏆 How to find heat of fusion. Heat of Sublimation. 20221010 Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry what is the sublimation process? Define phase transitions and phase transition temperatures. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. The reverse process of sublimation is called deposition, a process. The formation of frost is an example of. Its complement, deposition , is defined as a conversion. the. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.slideshare.net
Sublimation and deposition Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Its complement, deposition , is defined as a conversion. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. The reverse process of sublimation is called deposition, a process. the reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid state, bypassing the liquid. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.
From www.chemistrylearner.com
Heat (Enthalpy) of Fusion Definition, Equation, and Problems Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry Sublimation, including melting, freezing, and evaporation, is a form of. The enthalpy of sublimation, δhsub, is the energy required to convert one mole of a substance from the. what is the sublimation process? Solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. finally, sublimation. Explain The Process Of Fusion Evaporation And Sublimation In Aspects Of Chemistry.