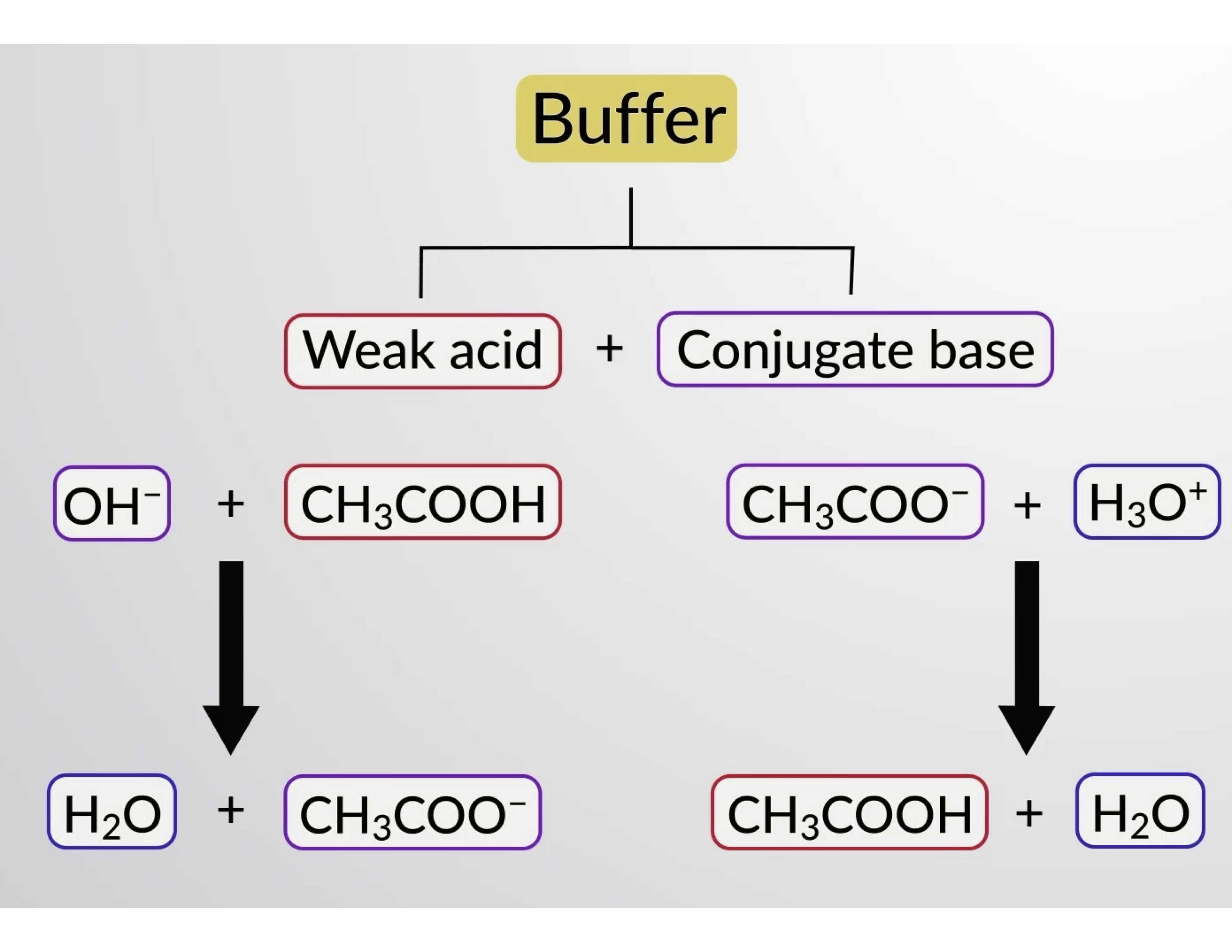
How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet . yes, the ph of the blood is controlled by the bicarbonate buffer. By interfering with the creation. a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. a buffer solution is typically composed of a weak acid and its conjugate base. The bicarbonate buffer system, the phosphate. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. It is the most important ecf. This is responsible for about 80% of extracellular buffering. how does the bicarbonate buffer system buffer a strong acid? By dissociating into carbon dioxide and water. There are three major buffer systems that are responsible for regulating blood ph: the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. the bicarbonate buffer system. By binding with hydrogen atoms.
from app.jove.com
There are three major buffer systems that are responsible for regulating blood ph: a buffer solution is typically composed of a weak acid and its conjugate base. a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. yes, the ph of the blood is controlled by the bicarbonate buffer. By dissociating into carbon dioxide and water. By binding with hydrogen atoms. By interfering with the creation. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. The bicarbonate buffer system, the phosphate. the bicarbonate buffer system.
Buffers, Buffer Components and Buffer Action Concept Chemistry JoVe
How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet There are three major buffer systems that are responsible for regulating blood ph: a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. By binding with hydrogen atoms. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. There are three major buffer systems that are responsible for regulating blood ph: It is the most important ecf. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. The bicarbonate buffer system, the phosphate. This is responsible for about 80% of extracellular buffering. yes, the ph of the blood is controlled by the bicarbonate buffer. the bicarbonate buffer system. By dissociating into carbon dioxide and water. By interfering with the creation. a buffer solution is typically composed of a weak acid and its conjugate base. how does the bicarbonate buffer system buffer a strong acid?
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet yes, the ph of the blood is controlled by the bicarbonate buffer. It is the most important ecf. By interfering with the creation. a buffer solution is typically composed of a weak acid and its conjugate base. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Carbonic AcidBicarbonate Buffering System PowerPoint How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By binding with hydrogen atoms. a buffer solution is typically composed of a weak acid and its conjugate base. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. the bicarbonate buffer system.. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideshare.net
Buffer system How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet a buffer solution is typically composed of a weak acid and its conjugate base. the bicarbonate buffer system. This is responsible for about 80% of extracellular buffering. how does the bicarbonate buffer system buffer a strong acid? The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. the added \(hcl\) (a strong. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Chapter 27 PowerPoint Presentation, free download ID3705071 How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. There are three major buffer systems that are responsible for regulating blood ph: a buffer solution is typically composed of a weak acid and its conjugate base. yes, the ph of the. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet This is responsible for about 80% of extracellular buffering. It is the most important ecf. the bicarbonate buffer system. a buffer solution is typically composed of a weak acid and its conjugate base. By interfering with the creation. yes, the ph of the blood is controlled by the bicarbonate buffer. The bicarbonate is regulated in the blood. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet the bicarbonate buffer system. By interfering with the creation. By binding with hydrogen atoms. a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. It is the most important ecf. The bicarbonate buffer system, the phosphate. There are three major buffer systems that. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From quizlet.com
The bicarbonate buffer system Diagram Quizlet How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet a buffer solution is typically composed of a weak acid and its conjugate base. There are three major buffer systems that are responsible for regulating blood ph: The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From courses.lumenlearning.com
10.5 Buffers The Basics of General, Organic, and Biological Chemistry How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet This is responsible for about 80% of extracellular buffering. The bicarbonate buffer system, the phosphate. how does the bicarbonate buffer system buffer a strong acid? a buffer solution is typically composed of a weak acid and its conjugate base. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From quizlet.com
Bicarbonate Buffer System Diagram Quizlet How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet yes, the ph of the blood is controlled by the bicarbonate buffer. The bicarbonate buffer system, the phosphate. By dissociating into carbon dioxide and water. This is responsible for about 80% of extracellular buffering. how does the bicarbonate buffer system buffer a strong acid? the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet yes, the ph of the blood is controlled by the bicarbonate buffer. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. a buffer solution is typically composed of a weak acid and. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. a buffer solution is typically composed of a weak acid and its conjugate base. By interfering with the creation. how does the bicarbonate buffer system buffer a strong acid? By dissociating into. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.youtube.com
Bicarbonate Buffer System YouTube How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By interfering with the creation. yes, the ph of the blood is controlled by the bicarbonate buffer. the bicarbonate buffer system. how does the bicarbonate buffer system buffer a strong acid? This is responsible for about 80% of extracellular buffering. The bicarbonate buffer system, the phosphate. a buffer has its highest capacity at equal concentrations of. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet yes, the ph of the blood is controlled by the bicarbonate buffer. The bicarbonate buffer system, the phosphate. By binding with hydrogen atoms. By interfering with the creation. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. This is responsible for about 80% of extracellular buffering. There are three major buffer systems that are. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet how does the bicarbonate buffer system buffer a strong acid? The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. There are three major buffer systems that are responsible for regulating blood ph: It is the most important ecf. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet This is responsible for about 80% of extracellular buffering. the bicarbonate buffer system. a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. a buffer solution is typically composed of a weak acid and its conjugate base. By binding with hydrogen atoms.. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet There are three major buffer systems that are responsible for regulating blood ph: a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. The bicarbonate buffer system, the phosphate. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From ar.inspiredpencil.com
Renal Buffer System How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet It is the most important ecf. There are three major buffer systems that are responsible for regulating blood ph: This is responsible for about 80% of extracellular buffering. By interfering with the creation. how does the bicarbonate buffer system buffer a strong acid? yes, the ph of the blood is controlled by the bicarbonate buffer. By dissociating into. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet the bicarbonate buffer system. This is responsible for about 80% of extracellular buffering. By dissociating into carbon dioxide and water. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. The bicarbonate buffer system,. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By interfering with the creation. This is responsible for about 80% of extracellular buffering. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. how does the bicarbonate buffer system buffer a strong acid? the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet This is responsible for about 80% of extracellular buffering. By interfering with the creation. It is the most important ecf. By binding with hydrogen atoms. There are three major buffer systems that are responsible for regulating blood ph: the bicarbonate buffer system. a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.sliderbase.com
Acid and Base Balance and Imbalance Presentation Chemistry How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet the bicarbonate buffer system. By dissociating into carbon dioxide and water. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. a buffer solution is typically composed of a weak acid and its. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From quizlet.com
buffering system Diagram Quizlet How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet The bicarbonate buffer system, the phosphate. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. It is the most important ecf. By interfering with the creation. The bicarbonate is regulated in the blood by. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From app.jove.com
Buffers, Buffer Components and Buffer Action Concept Chemistry JoVe How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By interfering with the creation. By dissociating into carbon dioxide and water. It is the most important ecf. a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. the bicarbonate buffer system. By binding with hydrogen atoms. There are three major buffer systems. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From in.pinterest.com
bicarbonate buffer system, example of multiple equilibria Teaching How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. By interfering with the creation. how does the bicarbonate buffer system buffer a strong acid? The bicarbonate buffer system, the phosphate. By binding with hydrogen atoms. By dissociating into carbon dioxide and water. a buffer solution is typically composed of a weak acid and. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From ar.inspiredpencil.com
Renal Buffer System How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet This is responsible for about 80% of extracellular buffering. The bicarbonate buffer system, the phosphate. There are three major buffer systems that are responsible for regulating blood ph: a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. It is the most important ecf.. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From fity.club
Buffer System How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. a buffer solution is typically composed of a weak acid and its conjugate base. how does the bicarbonate buffer system buffer a strong. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From study.com
Bicarbonate Buffer System Overview, Equation & Uses Video & Lesson How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By interfering with the creation. This is responsible for about 80% of extracellular buffering. a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base). How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideshare.net
Buffer system How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet a buffer solution is typically composed of a weak acid and its conjugate base. This is responsible for about 80% of extracellular buffering. By dissociating into carbon dioxide and water. yes, the ph of the blood is controlled by the bicarbonate buffer. It is the most important ecf. The bicarbonate buffer system, the phosphate. By interfering with the. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By interfering with the creation. It is the most important ecf. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. a buffer solution is typically composed of a weak acid and its conjugate. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From 2012books.lardbucket.org
Buffers How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By binding with hydrogen atoms. The bicarbonate buffer system, the phosphate. a buffer solution is typically composed of a weak acid and its conjugate base. By dissociating into carbon dioxide and water. By interfering with the creation. yes, the ph of the blood is controlled by the bicarbonate buffer. This is responsible for about 80% of extracellular buffering.. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. There are three major buffer systems that are responsible for regulating blood ph: a buffer solution is typically composed of a weak acid and its conjugate base. the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Chapter 27 PowerPoint Presentation, free download ID3705071 How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet a buffer has its highest capacity at equal concentrations of weak acid and conjugate base, when ph = pka p h = p k a. By interfering with the creation. how does the bicarbonate buffer system buffer a strong acid? The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. There are three major. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet how does the bicarbonate buffer system buffer a strong acid? the added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. yes, the ph of the blood is controlled by the bicarbonate buffer. It. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By binding with hydrogen atoms. yes, the ph of the blood is controlled by the bicarbonate buffer. a buffer solution is typically composed of a weak acid and its conjugate base. By interfering with the creation. By dissociating into carbon dioxide and water. The bicarbonate buffer system, the phosphate. The bicarbonate is regulated in the blood by sodium,. How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.
From www.numerade.com
SOLVED Q The buffer systems that are the most important in the How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet By dissociating into carbon dioxide and water. By interfering with the creation. The bicarbonate buffer system, the phosphate. It is the most important ecf. a buffer solution is typically composed of a weak acid and its conjugate base. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. the added \(hcl\) (a strong acid). How Does The Bicarbonate Buffer System Buffer A Strong Acid Quizlet.