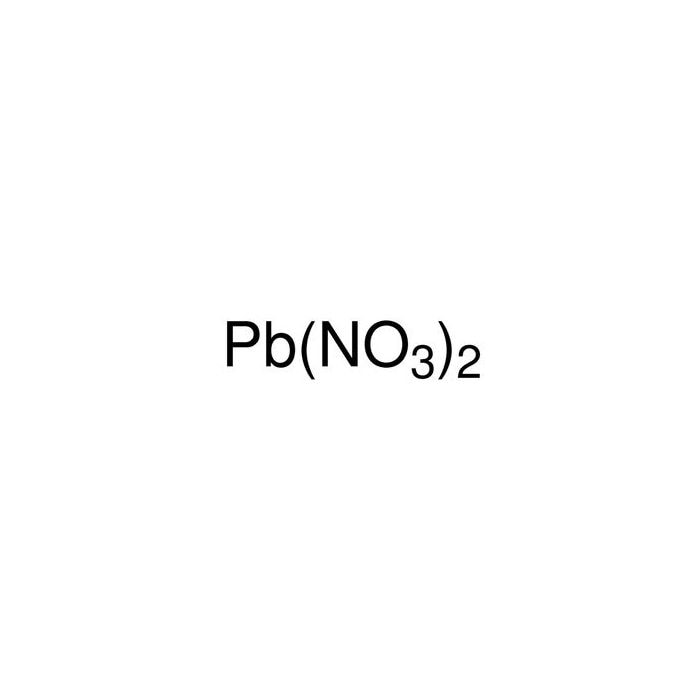
Lead Ii Nitrate And Water Equation . in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. It is an oxidizing agent and is used to make other. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. Metallic lead in the aqueous nitric acid, through the. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the.
from lab.honeywell.com
when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. Metallic lead in the aqueous nitric acid, through the. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water. It is an oxidizing agent and is used to make other. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid.
Lead(II) nitrate 228621 Honeywell Research Chemicals
Lead Ii Nitrate And Water Equation it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. It is an oxidizing agent and is used to make other. Metallic lead in the aqueous nitric acid, through the. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water.
From www.showme.com
Zinc and lead (II) nitrate react to form zinc nitrate lead Science Lead Ii Nitrate And Water Equation lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. in this video we will describe the equation pb (no3)2 + h2o and. Lead Ii Nitrate And Water Equation.
From lab.honeywell.com
Lead(II) nitrate 228621 Honeywell Research Chemicals Lead Ii Nitrate And Water Equation in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). It is an oxidizing agent and is used to make other. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead (ii) nitrate is obtained by the. Lead Ii Nitrate And Water Equation.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Lead Ii Nitrate And Water Equation it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. when you add nitric acid to lead(ii). Lead Ii Nitrate And Water Equation.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Lead Ii Nitrate And Water Equation it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. It is an oxidizing agent and is used to make other. in this video we will describe the equation pb (no3)2 + h2o and write what happens. Lead Ii Nitrate And Water Equation.
From www.coursehero.com
[Solved] Lead(II) nitrate reacts with potassium chromate to form lead Lead Ii Nitrate And Water Equation when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. Metallic lead in the aqueous nitric acid, through the. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). It is an oxidizing agent and is used to make other. lead(ii). Lead Ii Nitrate And Water Equation.
From www.meritnation.com
Valency of lead is 2 Valency of nitrate is 2 But chemical formula for Lead Ii Nitrate And Water Equation lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. in this video we. Lead Ii Nitrate And Water Equation.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Lead Ii Nitrate And Water Equation It is an oxidizing agent and is used to make other. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead. Lead Ii Nitrate And Water Equation.
From www.myxxgirl.com
Lead Ii Nitrate And Potassium Iodide My XXX Hot Girl Lead Ii Nitrate And Water Equation Metallic lead in the aqueous nitric acid, through the. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead (ii) nitrate is obtained by the dissolution. Lead Ii Nitrate And Water Equation.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead Ii Nitrate And Water Equation lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). It is an oxidizing agent and is used to make other. lead(ii) nitrate is the inorganic compound with chemical formula. Lead Ii Nitrate And Water Equation.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Ii Nitrate And Water Equation lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. . Lead Ii Nitrate And Water Equation.
From www.numerade.com
SOLVEDConvert the following into balanced equations (a) When lead(II Lead Ii Nitrate And Water Equation lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. Lead (ii). Lead Ii Nitrate And Water Equation.
From www.numerade.com
SOLVEDWhen a solution of lead(II) nitrate is mixed with a solution of Lead Ii Nitrate And Water Equation in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. lead. Lead Ii Nitrate And Water Equation.
From www.youtube.com
Lead II Nitrate Preparation and Properties YouTube Lead Ii Nitrate And Water Equation when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. Metallic lead in the aqueous nitric acid, through the. It is an oxidizing agent and is used to make other. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. lead nitrate is a nitrate of. Lead Ii Nitrate And Water Equation.
From www.youtube.com
lead (II) nitrate And Sodium Iodide Make Lead (II) iodide And sodium Lead Ii Nitrate And Water Equation lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. when you add. Lead Ii Nitrate And Water Equation.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Lead Ii Nitrate And Water Equation when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water. . Lead Ii Nitrate And Water Equation.
From www.youtube.com
Predict the Products Pb(NO3)2 + KI Lead (II) Nitrate + Potassium Lead Ii Nitrate And Water Equation it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric. Lead Ii Nitrate And Water Equation.
From questions.kunduz.com
Aluminum reacts with lead (II) nitrate a... Chemistry Lead Ii Nitrate And Water Equation in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. It is an oxidizing agent and is used. Lead Ii Nitrate And Water Equation.
From mccnsulting.web.fc2.com
What happens when lead nitrate is heated? Lead Ii Nitrate And Water Equation Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). It is an oxidizing agent and is used to. Lead Ii Nitrate And Water Equation.
From www.youtube.com
Equation for Pb(NO3)2 + H2O Lead (II) nitrate + Water YouTube Lead Ii Nitrate And Water Equation lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). It is an oxidizing agent. Lead Ii Nitrate And Water Equation.
From www.faqgear.com
What is the ionic equation of potassium iodide and lead nitrate The Lead Ii Nitrate And Water Equation in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. Metallic lead in the aqueous nitric acid, through. Lead Ii Nitrate And Water Equation.
From www.numerade.com
SOLVED A 12.505gram mixture contains Calcium nitrate and potassium Lead Ii Nitrate And Water Equation lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. it describes the formation of lead (ii) hydroxide, lead. Lead Ii Nitrate And Water Equation.
From www.toppr.com
If sodium chloride is added to silver nitrate solution a. Which Lead Ii Nitrate And Water Equation lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). lead(ii) nitrate is the inorganic compound with. Lead Ii Nitrate And Water Equation.
From www.youtube.com
How to Balance Pb(NO3)2 + Zn = Pb + Zn(NO3)2 Lead (II) nitrate + Zinc Lead Ii Nitrate And Water Equation in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a. Lead Ii Nitrate And Water Equation.
From www.coursehero.com
[Solved] If I start with 25.0 grams of lead (II) nitrate and 15.0 grams Lead Ii Nitrate And Water Equation Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3). Lead Ii Nitrate And Water Equation.
From wisc.pb.unizin.org
Solutions and Solubility (part 2) (M3Q2) UWMadison Chemistry 103/104 Lead Ii Nitrate And Water Equation lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. . Lead Ii Nitrate And Water Equation.
From www.numerade.com
SOLVED Solid nickel reacts with aqueous lead (II) nitrate to form Lead Ii Nitrate And Water Equation It is an oxidizing agent and is used to make other. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid.. Lead Ii Nitrate And Water Equation.
From www.coursehero.com
[Solved] Lead (II) nitrate volume (ml)=1.95, Molarity (m)= 0.268 Lead Ii Nitrate And Water Equation it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). Lead (ii). Lead Ii Nitrate And Water Equation.
From www.chegg.com
Solved An aqueous solution containing 9.56 g of lead(II) Lead Ii Nitrate And Water Equation Metallic lead in the aqueous nitric acid, through the. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. It is an. Lead Ii Nitrate And Water Equation.
From www.coursehero.com
[Solved] Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead Ii Nitrate And Water Equation Metallic lead in the aqueous nitric acid, through the. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and. Lead Ii Nitrate And Water Equation.
From www.teachoo.com
In the double displacement reaction b/w aqueo (MCQ) Class 10 Science Lead Ii Nitrate And Water Equation Metallic lead in the aqueous nitric acid, through the. Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. it describes the formation. Lead Ii Nitrate And Water Equation.
From www.numerade.com
SOLVEDLead(II) nitrate reacts with cesium sulfate in an aqueous Lead Ii Nitrate And Water Equation in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and is one of the few examples of a water. when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and. Lead Ii Nitrate And Water Equation.
From www.youtube.com
A Student carries out the reaction of lead nitrate to Lead Ii Nitrate And Water Equation It is an oxidizing agent and is used to make other. Metallic lead in the aqueous nitric acid, through the. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). when you add nitric acid to lead(ii) oxide, the reaction produces lead nitrate and water. lead. Lead Ii Nitrate And Water Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Lead Ii Nitrate And Water Equation It is an oxidizing agent and is used to make other. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead (ii). Lead (ii) nitrate is obtained by the dissolution of the pure lead i.e. lead(ii) nitrate is the inorganic compound with chemical formula pb(no 3) 2, and. Lead Ii Nitrate And Water Equation.
From www.numerade.com
SOLVEDDoes a reaction occur when aqueous solutions of sodium sulfate Lead Ii Nitrate And Water Equation lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Metallic lead in the aqueous nitric acid, through the. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or. Lead Ii Nitrate And Water Equation.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates Lead Ii Nitrate And Water Equation it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. It is an oxidizing agent and is used to make other. Metallic lead in the aqueous nitric acid, through the. in this video we will describe the equation pb (no3)2 + h2o and write what happens when pb (no3)2 (lead. Lead Ii Nitrate And Water Equation.