What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 . The $s$ subshell, which has 1 orbital. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. The second shell has 2 subshells: Therefore, maximum number of electrons will be two. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. Any orbital can have a maximum of 2 electrons with spin quantum. For $\ell=1$, $m_\ell$ has three possible values: Value of m = −1 represents one orbital. Thus, number of orbitals having n=3, l=1 and. The orbital associated with n = 3, l = 1 is 3p. According to pauli's exclusion principle, no two electrons can. We know that electrons have spin as ms =±1 2. N=3, l=1, the resultant subshell is 3p. Thus the $p$ subshell has three orbitals. The correct option is c 2.
from www.slideshare.net
Therefore, maximum number of electrons will be two. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. The correct option is c 2. The $s$ subshell, which has 1 orbital. N=3, l=1, the resultant subshell is 3p. We know that electrons have spin as ms =±1 2. The orbital associated with n = 3, l = 1 is 3p. According to pauli's exclusion principle, no two electrons can. The second shell has 2 subshells: Any orbital can have a maximum of 2 electrons with spin quantum.
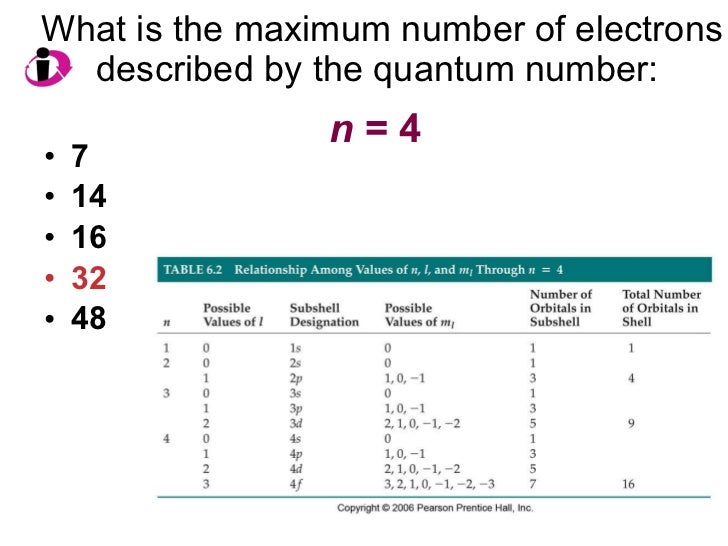
Chapter 6 Lecture Electrons in Atoms
What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N=3, l=1, the resultant subshell is 3p. The orbital associated with n = 3, l = 1 is 3p. We know that electrons have spin as ms =±1 2. Thus, number of orbitals having n=3, l=1 and. Thus the $p$ subshell has three orbitals. According to pauli's exclusion principle, no two electrons can. Therefore, maximum number of electrons will be two. Any orbital can have a maximum of 2 electrons with spin quantum. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. For $\ell=1$, $m_\ell$ has three possible values: The second shell has 2 subshells: The $s$ subshell, which has 1 orbital. N=3, l=1, the resultant subshell is 3p. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. Value of m = −1 represents one orbital. The correct option is c 2.
From www.nagwa.com
Question Video Identifying the Maximum Number of Electrons in the 1st What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The orbital associated with n = 3, l = 1 is 3p. Value of m = −1 represents one orbital. For $\ell=1$, $m_\ell$ has three possible values: N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. The second shell has 2 subshells: We know that electrons have spin as. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.toppr.com
0.6. What is the maximum number of electrons that can be associated What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N=3, l=1, the resultant subshell is 3p. Any orbital can have a maximum of 2 electrons with spin quantum. The orbital associated with n = 3, l = 1 is 3p. The correct option is c 2. For $\ell=1$, $m_\ell$ has three possible values: Thus, number of orbitals having n=3, l=1 and. Therefore, maximum number of electrons will be two.. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID2664384 What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N=3, l=1, the resultant subshell is 3p. Thus the $p$ subshell has three orbitals. According to pauli's exclusion principle, no two electrons can. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. N = 3, l = 1, m = 1 this implies it is an. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.numerade.com
SOLVED Q1What is the maximum number of electrons in an atom that can What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 For $\ell=1$, $m_\ell$ has three possible values: Thus, number of orbitals having n=3, l=1 and. The $s$ subshell, which has 1 orbital. We know that electrons have spin as ms =±1 2. N=3, l=1, the resultant subshell is 3p. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From ar.inspiredpencil.com
Number Of Electrons In Each Shell What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The $s$ subshell, which has 1 orbital. N=3, l=1, the resultant subshell is 3p. Thus the $p$ subshell has three orbitals. The orbital associated with n = 3, l = 1 is 3p. For $\ell=1$, $m_\ell$ has three possible values: Any orbital can have a maximum of 2 electrons with spin quantum. N = 3, l = 1, m =. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.nagwa.com
Question Video Determining the Maximum Number of Electrons in an S What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Any orbital can have a maximum of 2 electrons with spin quantum. Therefore, maximum number of electrons will be two. Thus the $p$ subshell has three orbitals. N=3, l=1, the resultant subshell is 3p. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. The orbital associated. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.toppr.com
What is the maximum number of electrons in an atom that can have the What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Value of m = −1 represents one orbital. Thus, number of orbitals having n=3, l=1 and. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. The $s$ subshell, which has 1 orbital. Thus the $p$ subshell has three orbitals. Therefore, maximum number of electrons will be. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.numerade.com
SOLVEDWhat is the maximum number of electrons in an atom that can have What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 For $\ell=1$, $m_\ell$ has three possible values: The correct option is c 2. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. The orbital associated with n = 3, l = 1 is 3p. N=3, l=1, the resultant subshell is 3p. Value of m = −1 represents one orbital.. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.toppr.com
Maximum number of electrons in an atom of the given element having l What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus, number of orbitals having n=3, l=1 and. The correct option is c 2. Therefore, maximum number of electrons will be two. Any orbital can have a maximum of 2 electrons with spin quantum. For $\ell=1$, $m_\ell$ has three possible values: We know that electrons have spin as ms =±1 2. Value of m = −1 represents one orbital. The. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.nagwa.com
Question Video Determining the Maximum Number of Electrons That Can What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N=3, l=1, the resultant subshell is 3p. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. The $s$ subshell, which has 1 orbital. Thus the $p$ subshell has three orbitals. The correct option is c 2. We know that electrons have spin as ms =±1 2. Study with quizlet. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.chegg.com
Solved How many electrons in an atom could have these sets What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus, number of orbitals having n=3, l=1 and. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. We know that electrons have spin as ms =±1 2. The $s$ subshell, which has 1 orbital. For $\ell=1$, $m_\ell$ has three possible values: The correct option is c 2. According to. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.toppr.com
Which one of the following has the maximum number of unpaired electrons? What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The orbital associated with n = 3, l = 1 is 3p. Value of m = −1 represents one orbital. We know that electrons have spin as ms =±1 2. Thus the $p$ subshell has three orbitals. N=3, l=1, the resultant subshell is 3p. The $s$ subshell, which has 1 orbital. Thus, number of orbitals having n=3, l=1 and. For. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From solvedlib.com
In one atom, what is the maximum number of electrons … SolvedLib What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The $s$ subshell, which has 1 orbital. Therefore, maximum number of electrons will be two. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. Any orbital can have a maximum of 2 electrons with spin quantum. For $\ell=1$, $m_\ell$ has three possible values: Thus the $p$. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.nagwa.com
Question Video Ranking Three Electrons from Lowest to Highest Energy What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus, number of orbitals having n=3, l=1 and. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. N=3, l=1, the resultant subshell is 3p. Any orbital can have a maximum of 2 electrons with spin quantum. The $s$ subshell, which has 1 orbital. Value of m = −1 represents. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.youtube.com
Maximum number of electrons in a subshell with l = 3 and n = 4 is YouTube What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus, number of orbitals having n=3, l=1 and. The correct option is c 2. The second shell has 2 subshells: We know that electrons have spin as ms =±1 2. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. Therefore, maximum number of electrons will be two. The orbital. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID3458314 What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus, number of orbitals having n=3, l=1 and. The correct option is c 2. Any orbital can have a maximum of 2 electrons with spin quantum. The orbital associated with n = 3, l = 1 is 3p. Value of m = −1 represents one orbital. Study with quizlet and memorize flashcards containing terms like what is the maximum number. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.toppr.com
What is the maximum number of electrons in an atom that can have the What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus the $p$ subshell has three orbitals. The orbital associated with n = 3, l = 1 is 3p. According to pauli's exclusion principle, no two electrons can. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. N = 3, l = 1, m = 1. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From oneclass.com
OneClass In a multielectron species, what is the maximum number of What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The orbital associated with n = 3, l = 1 is 3p. N=3, l=1, the resultant subshell is 3p. We know that electrons have spin as ms =±1 2. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. The correct option is c 2. Therefore, maximum number of electrons. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From byjus.com
what is the max. no. of electrons that can be associated with the What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N=3, l=1, the resultant subshell is 3p. The correct option is c 2. Thus the $p$ subshell has three orbitals. According to pauli's exclusion principle, no two electrons can. Therefore, maximum number of electrons will be two. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. Study with quizlet. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.slideserve.com
PPT Sorbitals PowerPoint Presentation, free download ID1830175 What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The correct option is c 2. Therefore, maximum number of electrons will be two. N=3, l=1, the resultant subshell is 3p. The orbital associated with n = 3, l = 1 is 3p. According to pauli's exclusion principle, no two electrons can. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From byjus.com
the total no. of valence electrons in 4.2g of N3 ion is? What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The orbital associated with n = 3, l = 1 is 3p. Thus, number of orbitals having n=3, l=1 and. According to pauli's exclusion principle, no two electrons can. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. Any orbital can have a maximum of 2 electrons with spin. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.slideshare.net
Chapter 6 Lecture Electrons in Atoms What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 According to pauli's exclusion principle, no two electrons can. The second shell has 2 subshells: Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. Value of m = −1 represents one orbital. The orbital associated with n = 3, l = 1 is 3p. Thus the. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.youtube.com
The total number of valence electrons in 4.2 g of N3ion is (NA is the What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus the $p$ subshell has three orbitals. The correct option is c 2. Thus, number of orbitals having n=3, l=1 and. N=3, l=1, the resultant subshell is 3p. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. The orbital associated with n = 3, l =. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From byjus.com
In an atom, for how many electrons, the quantum numbers will be, n=3, l What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 For $\ell=1$, $m_\ell$ has three possible values: Therefore, maximum number of electrons will be two. According to pauli's exclusion principle, no two electrons can. The orbital associated with n = 3, l = 1 is 3p. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. Thus, number of orbitals. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From vohobu-marria.blogspot.com
38 what is the maximum number of electrons that can occupy a box in an What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The second shell has 2 subshells: Therefore, maximum number of electrons will be two. The orbital associated with n = 3, l = 1 is 3p. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. According to pauli's exclusion principle, no two electrons can. The $s$. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From byjus.com
13. What is the maximum number of electrons in an atom in which the What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus the $p$ subshell has three orbitals. The correct option is c 2. The orbital associated with n = 3, l = 1 is 3p. Thus, number of orbitals having n=3, l=1 and. The second shell has 2 subshells: N=3, l=1, the resultant subshell is 3p. The $s$ subshell, which has 1 orbital. We know that electrons have spin as. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.youtube.com
12.1.4 State the maximum number of orbitals in a given energy level What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Therefore, maximum number of electrons will be two. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. According to pauli's exclusion principle, no two electrons can. For $\ell=1$, $m_\ell$ has three possible values: The orbital associated with n = 3, l = 1 is 3p. Any. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From byjus.com
how to calculate no. of electrons in given molecules?? What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The orbital associated with n = 3, l = 1 is 3p. Value of m = −1 represents one orbital. N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. The correct option is c 2. According to pauli's exclusion principle, no two electrons can. Study with quizlet and memorize. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.technocrazed.com
2.2 Quantum Physics What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Therefore, maximum number of electrons will be two. The $s$ subshell, which has 1 orbital. Thus, number of orbitals having n=3, l=1 and. The correct option is c 2. Any orbital can have a maximum of 2 electrons with spin quantum. Value of m = −1 represents one orbital. Thus the $p$ subshell has three orbitals. For $\ell=1$, $m_\ell$ has. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.nagwa.com
Question Video Using Quantum Numbers to Determine the Maximum Number What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N=3, l=1, the resultant subshell is 3p. The orbital associated with n = 3, l = 1 is 3p. For $\ell=1$, $m_\ell$ has three possible values: Any orbital can have a maximum of 2 electrons with spin quantum. We know that electrons have spin as ms =±1 2. Thus the $p$ subshell has three orbitals. According to pauli's exclusion principle,. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.youtube.com
What is the maximum number of electrons in the L shell of an atom is What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 The $s$ subshell, which has 1 orbital. The second shell has 2 subshells: According to pauli's exclusion principle, no two electrons can. Thus, number of orbitals having n=3, l=1 and. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. Any orbital can have a maximum of. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From ceaulups.blob.core.windows.net
Iron Has How Many Electrons In The Shells at Nicole Paige blog What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. The $s$ subshell, which has 1 orbital. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. According to pauli's exclusion principle, no two electrons can. We. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.nagwa.com
Question Video Determining How Many Electrons in an Atom Can Have a What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 Thus, number of orbitals having n=3, l=1 and. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can be identified with each of. For $\ell=1$, $m_\ell$ has three possible values: N = 3, l = 1, m = 1 this implies it is an orbital of 3 p sub shell. N=3, l=1,. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.youtube.com
Number of Electrons in Lithium (Li) YouTube What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N=3, l=1, the resultant subshell is 3p. For $\ell=1$, $m_\ell$ has three possible values: Value of m = −1 represents one orbital. The orbital associated with n = 3, l = 1 is 3p. Thus the $p$ subshell has three orbitals. Any orbital can have a maximum of 2 electrons with spin quantum. The correct option is c 2. The. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.
From www.slideshare.net
Orbitals electronconfiguration What Is The Maximum Number Of Electrons In N=3 L=1 M=-1 N=3, l=1, the resultant subshell is 3p. We know that electrons have spin as ms =±1 2. Any orbital can have a maximum of 2 electrons with spin quantum. For $\ell=1$, $m_\ell$ has three possible values: The correct option is c 2. Study with quizlet and memorize flashcards containing terms like what is the maximum number of electrons that can. What Is The Maximum Number Of Electrons In N=3 L=1 M=-1.