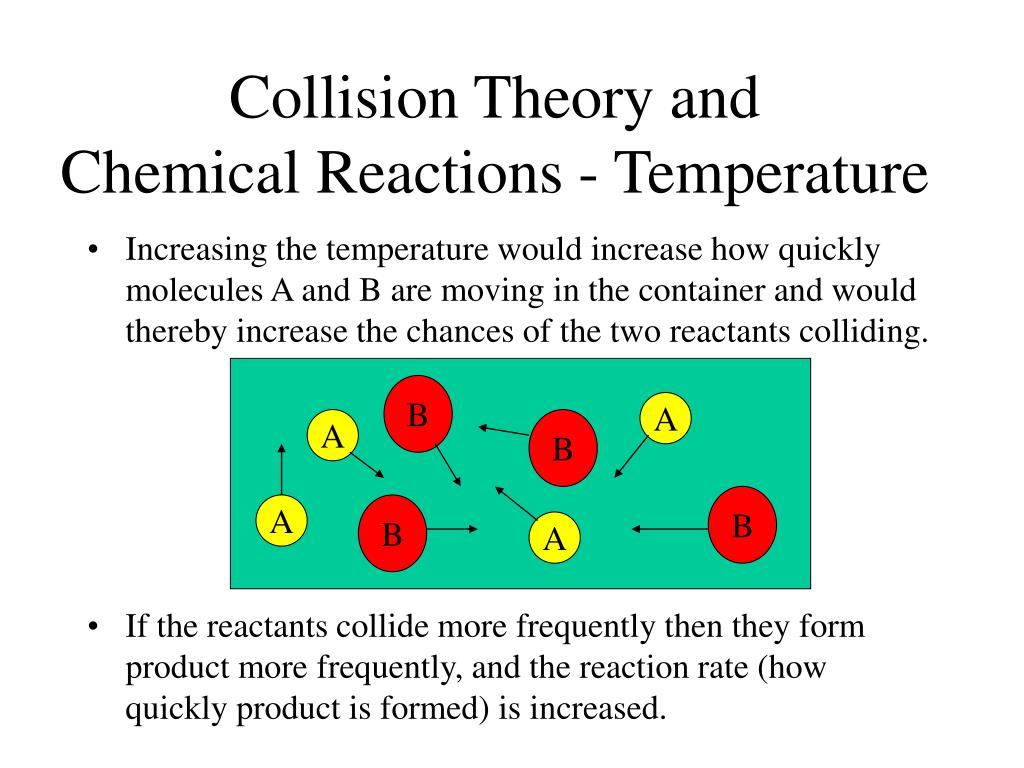
Collision Theory Effect Of Temperature . increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. With an increase in the concentration of. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. collision theory and temperature effects on rates. Kinetic theory says that molecules are in constant motion. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. collision theory explains why most reaction rates increase as concentrations increase.
from www.slideserve.com
increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. collision theory explains why most reaction rates increase as concentrations increase. collision theory and temperature effects on rates. With an increase in the concentration of. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. Kinetic theory says that molecules are in constant motion.
PPT Chemical Reactions and Collision Theory PowerPoint Presentation
Collision Theory Effect Of Temperature collision theory explains why most reaction rates increase as concentrations increase. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. collision theory and temperature effects on rates. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. collision theory explains why most reaction rates increase as concentrations increase. Kinetic theory says that molecules are in constant motion. increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. With an increase in the concentration of. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Theory Effect Of Temperature For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. With an increase in the concentration of. collision theory explains why most reaction rates increase as concentrations increase. Kinetic theory says that molecules are in constant motion. the collision model explains why chemical reactions often occur. Collision Theory Effect Of Temperature.
From www.numerade.com
SOLVEDAccording to the collision theory of reaction rates, an increase Collision Theory Effect Of Temperature collision theory explains why most reaction rates increase as concentrations increase. With an increase in the concentration of. Kinetic theory says that molecules are in constant motion. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. increasing the temperature increases reaction rates because of the disproportionately large increase. Collision Theory Effect Of Temperature.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Theory Effect Of Temperature the collision model explains why chemical reactions often occur more rapidly at higher temperatures. increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. collision theory and temperature effects. Collision Theory Effect Of Temperature.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID9507407 Collision Theory Effect Of Temperature For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. Kinetic theory says that molecules are in constant motion. increasing the temperature increases reaction rates because of the disproportionately. Collision Theory Effect Of Temperature.
From www.slideserve.com
PPT Unit 9 Reaction Rates and Equilibrium PowerPoint Presentation Collision Theory Effect Of Temperature use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. With an increase in the concentration of. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. collision theory explains why most reaction rates increase as concentrations increase. . Collision Theory Effect Of Temperature.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Theory Effect Of Temperature collision theory and temperature effects on rates. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. use the postulates of collision theory to explain the effects. Collision Theory Effect Of Temperature.
From www.slideshare.net
(Chapter 7) And Equilibrium Collision Theory Effect Of Temperature Kinetic theory says that molecules are in constant motion. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. With an increase in the concentration of. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. collision theory and temperature effects on rates.. Collision Theory Effect Of Temperature.
From www.tec-science.com
Temperature and particle motion tecscience Collision Theory Effect Of Temperature the collision model explains why chemical reactions often occur more rapidly at higher temperatures. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. With an increase in the concentration of. increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions.. Collision Theory Effect Of Temperature.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation ID4450666 Collision Theory Effect Of Temperature the collision model explains why chemical reactions often occur more rapidly at higher temperatures. increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. collision theory explains why most reaction rates increase as concentrations increase. collision theory provides a simple but effective explanation for the effect of. Collision Theory Effect Of Temperature.
From www.youtube.com
Collision Theory and Reaction Rate // HSC Chemistry YouTube Collision Theory Effect Of Temperature collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. Kinetic theory says that molecules are in constant motion. With an increase in the concentration of. collision theory explains why most reaction. Collision Theory Effect Of Temperature.
From chem.libretexts.org
6.4 The Effect of Temperature on Reaction Rate Chemistry LibreTexts Collision Theory Effect Of Temperature collision theory and temperature effects on rates. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature. Collision Theory Effect Of Temperature.
From www.slideserve.com
PPT Chemical Reactions and Collision Theory PowerPoint Presentation Collision Theory Effect Of Temperature Kinetic theory says that molecules are in constant motion. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. collision theory and temperature effects on rates. use. Collision Theory Effect Of Temperature.
From classzonestewart.z19.web.core.windows.net
What Is The Collision Theory Collision Theory Effect Of Temperature For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. collision theory explains why most reaction rates increase as concentrations increase. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. Kinetic theory says that molecules are in constant motion. use. Collision Theory Effect Of Temperature.
From wiringfixsprang.z19.web.core.windows.net
Parts Of Collision Theory Collision Theory Effect Of Temperature increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. collision theory and temperature effects on rates. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. the collision model explains why chemical reactions often occur more rapidly at higher. Collision Theory Effect Of Temperature.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 Collision Theory Effect Of Temperature With an increase in the concentration of. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. Kinetic theory says that molecules are in constant motion. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. increasing the temperature increases reaction rates. Collision Theory Effect Of Temperature.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Theory Effect Of Temperature use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. With an increase in the concentration of. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. collision theory and temperature effects on rates. use the postulates of. Collision Theory Effect Of Temperature.
From slideplayer.com
Lesson 3 Collision Theory. ppt download Collision Theory Effect Of Temperature With an increase in the concentration of. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Kinetic theory says that molecules are in constant motion. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. increasing the. Collision Theory Effect Of Temperature.
From www.youtube.com
Collision Theory. Effect of temperature and concentration on rate Collision Theory Effect Of Temperature increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. Kinetic theory says that molecules are in constant motion. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. use the postulates of collision theory to explain the effects of. Collision Theory Effect Of Temperature.
From app.jove.com
Effect of Temperature on Reaction Rate, Arrhenius Equation and Collision Theory Effect Of Temperature the collision model explains why chemical reactions often occur more rapidly at higher temperatures. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. With an increase in the concentration of. increasing the temperature increases reaction rates because of the disproportionately large increase in the number. Collision Theory Effect Of Temperature.
From www.youtube.com
Effect of Temperature on Rates of a Reversible Reaction at Equilibrium Collision Theory Effect Of Temperature increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. With an increase in the concentration of. Kinetic theory says that molecules are in constant motion. collision theory explains why most reaction rates increase as concentrations increase. For example, the reaction rates of many reactions that occur at room. Collision Theory Effect Of Temperature.
From slidetodoc.com
Collision Theory Temperature w Increasing the temperature increases Collision Theory Effect Of Temperature increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. collision theory explains why most reaction rates increase as concentrations increase. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. Kinetic theory says that molecules are in constant motion. For. Collision Theory Effect Of Temperature.
From www.youtube.com
How Collision Theory Relates to Equilibrium // HSC Chemistry YouTube Collision Theory Effect Of Temperature collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. . Collision Theory Effect Of Temperature.
From classnotes.org.in
Collision Theory Chemical Chemistry, Class 12 Collision Theory Effect Of Temperature collision theory and temperature effects on rates. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. With an increase in the concentration of. collision theory explains why most reaction rates increase as concentrations increase. collision theory provides a simple but effective explanation for the. Collision Theory Effect Of Temperature.
From mungfali.com
Maxwell Boltzmann Distribution Temperature Collision Theory Effect Of Temperature collision theory explains why most reaction rates increase as concentrations increase. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. With an increase in the concentration of. collision theory provides a. Collision Theory Effect Of Temperature.
From www.ck12.org
Collision Theory CK12 Foundation Collision Theory Effect Of Temperature use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. With an increase in the concentration of. collision. Collision Theory Effect Of Temperature.
From www.slideserve.com
PPT 6 PowerPoint Presentation, free download ID3183574 Collision Theory Effect Of Temperature For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Kinetic theory says that molecules are in constant motion. increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. use the postulates of collision theory to explain. Collision Theory Effect Of Temperature.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Collision Theory Effect Of Temperature With an increase in the concentration of. collision theory and temperature effects on rates. collision theory explains why most reaction rates increase as concentrations increase. Kinetic theory says that molecules are in constant motion. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. increasing the temperature increases. Collision Theory Effect Of Temperature.
From slideplayer.com
Chemical Reactions and Collision Theory ppt download Collision Theory Effect Of Temperature With an increase in the concentration of. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of. Collision Theory Effect Of Temperature.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Collision Theory Effect Of Temperature collision theory explains why most reaction rates increase as concentrations increase. increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. With an increase in the concentration of. the collision model explains why chemical reactions often occur more rapidly at higher temperatures. use the postulates of collision. Collision Theory Effect Of Temperature.
From www.youtube.com
Temperature dependence theory of rate constant Arrhenius Equation Collision Theory Effect Of Temperature collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. collision theory explains why most reaction rates increase as concentrations increase. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. Kinetic theory says that molecules are in constant motion. With an. Collision Theory Effect Of Temperature.
From thescienceandmathszone.com
Simple Collision Theory The Science and Maths Zone Collision Theory Effect Of Temperature use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. With an increase in the concentration of. collision theory and temperature effects on rates. Kinetic theory says that molecules. Collision Theory Effect Of Temperature.
From www.slideserve.com
PPT 3.1 Collision Theory PowerPoint Presentation, free download ID Collision Theory Effect Of Temperature increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Kinetic theory says that molecules are in constant motion. collision theory provides a simple but effective explanation. Collision Theory Effect Of Temperature.
From slideplayer.com
Chemical and Stability ppt download Collision Theory Effect Of Temperature use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. With an increase in the concentration of. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Kinetic theory says that molecules are in constant motion. collision theory explains. Collision Theory Effect Of Temperature.
From slideplayer.com
Collision Theory. ppt download Collision Theory Effect Of Temperature increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. Kinetic theory says that molecules are in constant motion. use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. With an increase in the concentration of. collision theory explains why most. Collision Theory Effect Of Temperature.
From www.youtube.com
Collision theory temperature YouTube Collision Theory Effect Of Temperature With an increase in the concentration of. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. the collision model explains why chemical reactions often occur more rapidly at higher. Collision Theory Effect Of Temperature.