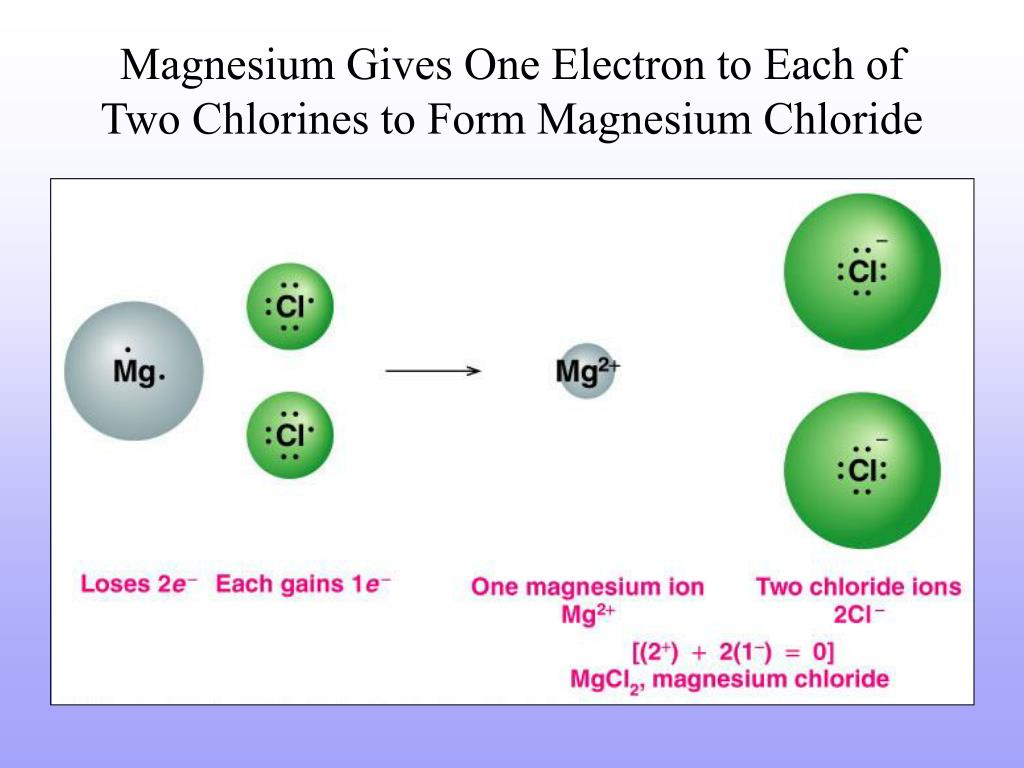
Magnesium Chloride Valence . Magnesium is in group 2 of the periodic table. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — let's dive into drawing the lewis structure of mgcl2: — magnesium is an alkali metal found in group 2 of the periodic table. A magnesium atom will lose 2 electrons to form a stable. the ionic bond formation for magnesium chloride. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. It has an electronic configuration of [ne]3s2. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. — valence electrons: The electron configuration of mg is. Now, let’s calculate the same for chlorine.
from www.slideserve.com
— magnesium is an alkali metal found in group 2 of the periodic table. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Now, let’s calculate the same for chlorine. — valence electrons: — let's dive into drawing the lewis structure of mgcl2: Magnesium is in group 2 of the periodic table. the ionic bond formation for magnesium chloride. A magnesium atom will lose 2 electrons to form a stable.
PPT Valence Electrons PowerPoint Presentation, free download ID651377
Magnesium Chloride Valence Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. — valence electrons: 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. The electron configuration of mg is. — let's dive into drawing the lewis structure of mgcl2: Now, let’s calculate the same for chlorine. — magnesium is an alkali metal found in group 2 of the periodic table. A magnesium atom will lose 2 electrons to form a stable. the ionic bond formation for magnesium chloride. It has an electronic configuration of [ne]3s2. Magnesium is in group 2 of the periodic table.
From www.anyrgb.com
Magnesium Fluoride, magnesium Bromide, magnesium Iodide, magnesium Magnesium Chloride Valence Magnesium is in group 2 of the periodic table. The electron configuration of mg is. the ionic bond formation for magnesium chloride. A magnesium atom will lose 2 electrons to form a stable. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. — valence electrons: It has an electronic configuration. Magnesium Chloride Valence.
From www.youtube.com
Valence Electrons for Magnesium (Mg) YouTube Magnesium Chloride Valence It has an electronic configuration of [ne]3s2. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Now, let’s calculate the same for chlorine. — magnesium is an alkali metal found in group 2 of the periodic table. Magnesium is in group 2 of the periodic table. — valence electrons: A. Magnesium Chloride Valence.
From socratic.org
Question 15936 + Example Magnesium Chloride Valence — magnesium is an alkali metal found in group 2 of the periodic table. the ionic bond formation for magnesium chloride. — valence electrons: The electron configuration of mg is. A magnesium atom will lose 2 electrons to form a stable. It has an electronic configuration of [ne]3s2. — let's dive into drawing the lewis structure. Magnesium Chloride Valence.
From www.amazon.co.uk
Magnesium Chloride, 454g, Hexahydrate, Pharmaceutical Grade, Crystal Magnesium Chloride Valence The electron configuration of mg is. — magnesium is an alkali metal found in group 2 of the periodic table. — valence electrons: Now, let’s calculate the same for chlorine. — let's dive into drawing the lewis structure of mgcl2: A magnesium atom will lose 2 electrons to form a stable. the ionic bond formation for. Magnesium Chloride Valence.
From www.vrogue.co
How Can I Draw An Orbital Diagram For Chloride Ions vrogue.co Magnesium Chloride Valence the ionic bond formation for magnesium chloride. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Now, let’s calculate the same for chlorine. Magnesium is in group 2 of the periodic table. — let's dive into drawing the lewis structure of mgcl2: — valence electrons: It has an electronic. Magnesium Chloride Valence.
From cymitquimica.com
Magnesium chloride 3DFM05982 CymitQuimica Magnesium Chloride Valence — magnesium is an alkali metal found in group 2 of the periodic table. — valence electrons: A magnesium atom will lose 2 electrons to form a stable. — let's dive into drawing the lewis structure of mgcl2: Magnesium is in group 2 of the periodic table. the ionic bond formation for magnesium chloride. It has. Magnesium Chloride Valence.
From www.drugs.com
Magnesium Chloride Injection FDA prescribing information, side Magnesium Chloride Valence The electron configuration of mg is. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. It. Magnesium Chloride Valence.
From www.slideshare.net
Metals and non metals Magnesium Chloride Valence Magnesium is in group 2 of the periodic table. — let's dive into drawing the lewis structure of mgcl2: — valence electrons: The electron configuration of mg is. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms.. Magnesium Chloride Valence.
From www.showme.com
ShowMe magnesium chloride Magnesium Chloride Valence Now, let’s calculate the same for chlorine. A magnesium atom will lose 2 electrons to form a stable. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. It has. Magnesium Chloride Valence.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Chloride Valence — valence electrons: — let's dive into drawing the lewis structure of mgcl2: — magnesium is an alkali metal found in group 2 of the periodic table. the ionic bond formation for magnesium chloride. A magnesium atom will lose 2 electrons to form a stable. — mg forms ionic bond to cl by donating its. Magnesium Chloride Valence.
From www.numerade.com
SOLVED What will cause more Magnesium to be dissolved in a solution of Magnesium Chloride Valence — valence electrons: the ionic bond formation for magnesium chloride. — magnesium is an alkali metal found in group 2 of the periodic table. Magnesium is in group 2 of the periodic table. The electron configuration of mg is. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. . Magnesium Chloride Valence.
From medlibrary.org
Magnesium Chloride (Mylan Institutional LLC) FDA Package Insert Magnesium Chloride Valence — magnesium is an alkali metal found in group 2 of the periodic table. — let's dive into drawing the lewis structure of mgcl2: A magnesium atom will lose 2 electrons to form a stable. It has an electronic configuration of [ne]3s2. the ionic bond formation for magnesium chloride. — mg forms ionic bond to cl. Magnesium Chloride Valence.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride Valence Magnesium is in group 2 of the periodic table. the ionic bond formation for magnesium chloride. The electron configuration of mg is. — magnesium is an alkali metal found in group 2 of the periodic table. — valence electrons: Now, let’s calculate the same for chlorine. — mg forms ionic bond to cl by donating its. Magnesium Chloride Valence.
From www.freshhealthnutrition.com
Best Naturals Magnesium Chloride 520 mg 120 Tablets Fresh Health Magnesium Chloride Valence — valence electrons: It has an electronic configuration of [ne]3s2. — let's dive into drawing the lewis structure of mgcl2: A magnesium atom will lose 2 electrons to form a stable. Now, let’s calculate the same for chlorine. Magnesium is in group 2 of the periodic table. Therefore, a single magnesium atom contributes 2 x 1 = 2. Magnesium Chloride Valence.
From www.pngegg.com
Lewis structure Magnesium chloride Electron Diagram, Dot, text Magnesium Chloride Valence The electron configuration of mg is. — valence electrons: — magnesium is an alkali metal found in group 2 of the periodic table. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. A magnesium atom will lose 2 electrons to form a stable. . Magnesium Chloride Valence.
From pubs.rsc.org
Elucidating the structure of the magnesium aluminum chloride complex Magnesium Chloride Valence The electron configuration of mg is. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. — magnesium is an alkali metal found in group 2 of the periodic table. the ionic bond formation for magnesium chloride. — let's dive into drawing the lewis structure of mgcl2: 93 rows — you may assume. Magnesium Chloride Valence.
From chiangmaiplaces.net
How Is Mgcl2 An Ionic Compound? The 18 Detailed Answer Magnesium Chloride Valence 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. It has an electronic configuration of [ne]3s2. Now, let’s calculate the same for chlorine. the ionic bond formation for magnesium chloride. —. Magnesium Chloride Valence.
From www.alamy.com
3D image of Magnesium chloride skeletal formula molecular chemical Magnesium Chloride Valence Magnesium is in group 2 of the periodic table. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. — let's dive into drawing the lewis structure of mgcl2:. Magnesium Chloride Valence.
From www.toppr.com
Draw electron dot representation for the formation of magnesium chloride. Magnesium Chloride Valence The electron configuration of mg is. Magnesium is in group 2 of the periodic table. A magnesium atom will lose 2 electrons to form a stable. Now, let’s calculate the same for chlorine. the ionic bond formation for magnesium chloride. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an. Magnesium Chloride Valence.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Magnesium Chloride Valence — magnesium is an alkali metal found in group 2 of the periodic table. the ionic bond formation for magnesium chloride. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — valence electrons: The electron configuration of mg is. A magnesium atom will. Magnesium Chloride Valence.
From www.youtube.com
Formation Of Magnesium Chloride YouTube Magnesium Chloride Valence Now, let’s calculate the same for chlorine. — valence electrons: Magnesium is in group 2 of the periodic table. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. — magnesium is an alkali metal found in group 2 of the periodic table. It has an electronic configuration of [ne]3s2. The electron configuration of mg. Magnesium Chloride Valence.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Chloride Valence the ionic bond formation for magnesium chloride. It has an electronic configuration of [ne]3s2. — valence electrons: Now, let’s calculate the same for chlorine. The electron configuration of mg is. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — mg forms ionic. Magnesium Chloride Valence.
From hanenhuusholli.blogspot.com
Dot Diagram Of Magnesium Chloride Hanenhuusholli Magnesium Chloride Valence Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. A magnesium atom will lose 2 electrons. Magnesium Chloride Valence.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Magnesium Chloride Valence — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. A magnesium atom will lose 2 electrons to form a stable. Now, let’s calculate the same for chlorine. — magnesium is an alkali metal found in group 2 of the periodic table. — let's dive into drawing the lewis structure of. Magnesium Chloride Valence.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID651377 Magnesium Chloride Valence the ionic bond formation for magnesium chloride. — valence electrons: A magnesium atom will lose 2 electrons to form a stable. The electron configuration of mg is. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which. Magnesium Chloride Valence.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Magnesium Chloride Valence — valence electrons: — magnesium is an alkali metal found in group 2 of the periodic table. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Now, let’s calculate the same for chlorine. Magnesium is in group 2 of the periodic table. A magnesium atom will lose 2 electrons to. Magnesium Chloride Valence.
From www.winharvest.com.au
Magnesium Chloride Winharvest Magnesium Chloride Valence Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. the ionic bond formation for magnesium chloride. Magnesium is in group 2 of the periodic table. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Now, let’s calculate the same for chlorine.. Magnesium Chloride Valence.
From pediaa.com
Difference Between Magnesium Chloride and Magnesium Sulfate Magnesium Chloride Valence — let's dive into drawing the lewis structure of mgcl2: Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. — magnesium is an alkali metal found in group 2 of the periodic table. Magnesium is in group 2 of the periodic table. — mg forms ionic bond to cl by donating its valence. Magnesium Chloride Valence.
From beamzen.com
Magnesium Chloride Supplement Capsules BeamZen Magnesium Chloride Valence — magnesium is an alkali metal found in group 2 of the periodic table. — let's dive into drawing the lewis structure of mgcl2: the ionic bond formation for magnesium chloride. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. Magnesium is in group 2 of the periodic table. — valence electrons:. Magnesium Chloride Valence.
From www.pinterest.co.uk
This is the electron configuration for sodium. Like ALL of the other Magnesium Chloride Valence Magnesium is in group 2 of the periodic table. A magnesium atom will lose 2 electrons to form a stable. It has an electronic configuration of [ne]3s2. — let's dive into drawing the lewis structure of mgcl2: Now, let’s calculate the same for chlorine. 93 rows — you may assume the valences of the chemical elements—the number of. Magnesium Chloride Valence.
From www.showme.com
ShowMe magnesium chloride Magnesium Chloride Valence It has an electronic configuration of [ne]3s2. Now, let’s calculate the same for chlorine. — let's dive into drawing the lewis structure of mgcl2: The electron configuration of mg is. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — valence electrons: A magnesium. Magnesium Chloride Valence.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Chloride Valence — valence electrons: the ionic bond formation for magnesium chloride. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The electron configuration of mg is. A magnesium. Magnesium Chloride Valence.
From whatsinsight.org
Is MgCl2 Ionic or Covalent? What's Insight Magnesium Chloride Valence — valence electrons: — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Magnesium is in group 2 of the periodic table. The electron configuration of mg is. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Therefore,. Magnesium Chloride Valence.
From galvinconanstuart.blogspot.com
Dot Diagram Of Magnesium Chloride General Wiring Diagram Magnesium Chloride Valence Magnesium is in group 2 of the periodic table. — mg forms ionic bond to cl by donating its valence electrons to two cl atoms. The electron configuration of mg is. Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. — let's dive into drawing the lewis structure of mgcl2: 93 rows —. Magnesium Chloride Valence.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Chloride Valence Therefore, a single magnesium atom contributes 2 x 1 = 2 valence electrons. — valence electrons: It has an electronic configuration of [ne]3s2. The electron configuration of mg is. A magnesium atom will lose 2 electrons to form a stable. — magnesium is an alkali metal found in group 2 of the periodic table. — mg forms. Magnesium Chloride Valence.