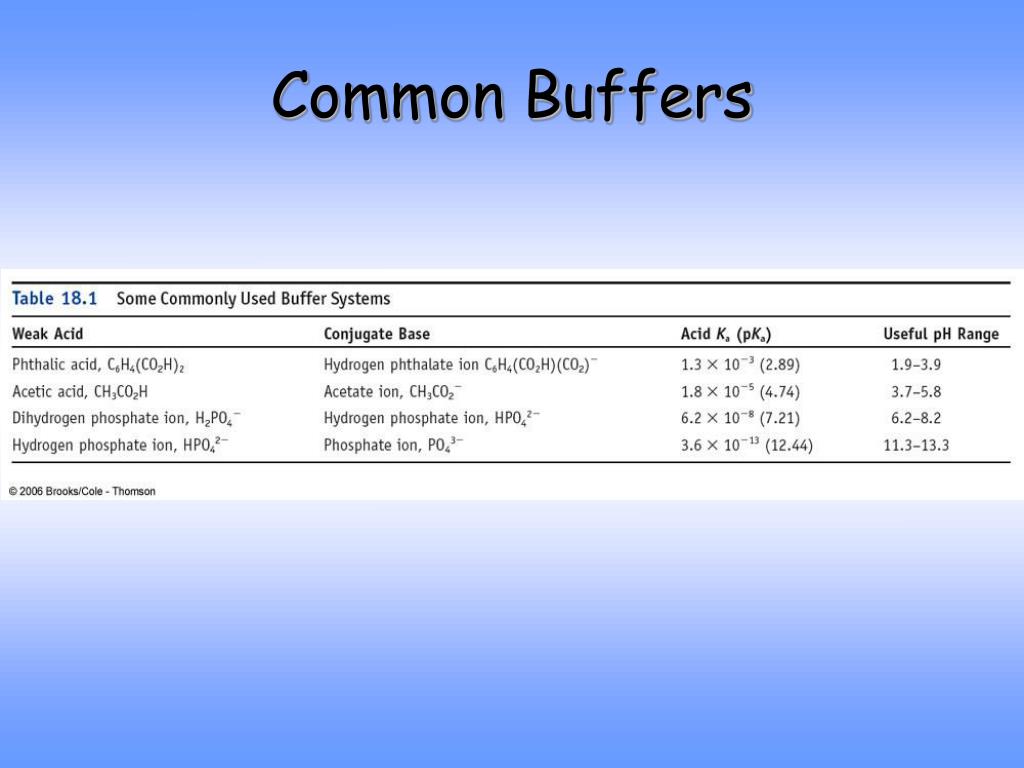
Common Buffer . an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes.
from www.slideserve.com
a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes.
PPT Chapter 18 Other Aspects of Aqueous Equilibria PowerPoint
Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Common Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. This characteristic makes. Common Buffer.
From fyonnnmfq.blob.core.windows.net
Uses Of Buffers In Biochemistry at Grace McMillan blog Common Buffer an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes. Common Buffer.
From www.youtube.com
Preparation of common buffers Do's and Don'ts YouTube Common Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. This characteristic makes buffers important in biological and chemical applications. Common Buffer.
From hxebwfdfq.blob.core.windows.net
Buffers In Real Life Examples at Carl McCarty blog Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution. Common Buffer.
From www.biobasic.com
Common Buffers Biological Buffers Biochemicals Products Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the. Common Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID3308656 Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. a buffer is a solution that maintains the. Common Buffer.
From www.scribd.com
Preparation and Characterization of Common Pharmaceutical Buffers PDF Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. This. Common Buffer.
From www.numerade.com
SOLVED(12 points) Given the following table of pka for some common Common Buffer an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the buffer capacity is the. Common Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. a buffer is a solution that can resist ph change upon the addition of an acidic or. Common Buffer.
From pubs.acs.org
Interactions of Common Biological Buffers with Iron Oxide Nanoparticles Common Buffer the following tables can help you navigate preparation of many common buffer solutions by ph and pka. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the buffer capacity is the amount of acid or base. Common Buffer.
From www.slideserve.com
PPT Ionic Equilibria 離子平衡 Part II Buffers 緩衝液 and Titration Curves Common Buffer an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. a buffer is a solution that can resist ph change upon the addition of an acidic. Common Buffer.
From fyonnnmfq.blob.core.windows.net
Uses Of Buffers In Biochemistry at Grace McMillan blog Common Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the following tables can help you navigate preparation of. Common Buffer.
From currentprotocols.onlinelibrary.wiley.com
Common Buffers and Stock Solutions 2001 Current Protocols in Food Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the. Common Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer. Common Buffer.
From www.chegg.com
Solved Table of Common Buffers\table[[Buffer,pK1,pK2,pK3 Common Buffer the following tables can help you navigate preparation of many common buffer solutions by ph and pka. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes. Common Buffer.
From www.researchgate.net
List of common buffer components and their maximum allowed Common Buffer an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph. Common Buffer.
From www.researchgate.net
Compatibility of six candidates with common buffer components. 1D 19 F Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the buffer capacity is the amount. Common Buffer.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or. Common Buffer.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. the buffer capacity is the. Common Buffer.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. a buffer is a solution that maintains the stability of a system’s. Common Buffer.
From www.slideserve.com
PPT Chapter 18 Other Aspects of Aqueous Equilibria PowerPoint Common Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the following tables can help you navigate preparation of. Common Buffer.
From www.slideserve.com
PPT Chapter 18 Other Aspects of Aqueous Equilibria PowerPoint Common Buffer an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the buffer capacity is the amount of acid. Common Buffer.
From www.slideserve.com
PPT AcidBase Equilibria PowerPoint Presentation, free download ID Common Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph. Common Buffer.
From www.slideserve.com
PPT DEFINITIONS OF ACIDS & BASES PowerPoint Presentation, free Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the buffer capacity is the amount. Common Buffer.
From www.researchgate.net
Schematic comparing metamorphic buffer layer composition profiles for Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. a buffer is a solution. Common Buffer.
From www.youtube.com
Construction of common bus system using tristate buffer in computer Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before. Common Buffer.
From www.slideserve.com
PPT Exploiting Format String Vulnerabilities PowerPoint Presentation Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. a buffer is. Common Buffer.
From www.youtube.com
Common Ion Effect and Buffer Solutions YouTube Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. . Common Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Common Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. a. Common Buffer.
From cosmoposts.weebly.com
Common Buffer Compounds Used In Biology cosmoposts Common Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. a buffer is a solution. Common Buffer.
From www.slideserve.com
PPT Weak acids and buffers PowerPoint Presentation, free download Common Buffer an example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a. Common Buffer.
From answers.flexsim.com
How to create common buffer in object process flow? FlexSim Community Common Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes.. Common Buffer.
From hxerjrfyx.blob.core.windows.net
What Is A Buffer Anatomy at Alisa Williams blog Common Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the following tables can help you navigate preparation of many common buffer solutions by ph and pka. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the buffer capacity. Common Buffer.
From hxeckoohh.blob.core.windows.net
Common Protein Buffers at Angela Watkins blog Common Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s. Common Buffer.
From exyfnmepw.blob.core.windows.net
Common Molecular Biology Buffers at Donna Davis blog Common Buffer the following tables can help you navigate preparation of many common buffer solutions by ph and pka. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. a buffer is a solution that maintains the stability of a system’s ph level. Common Buffer.