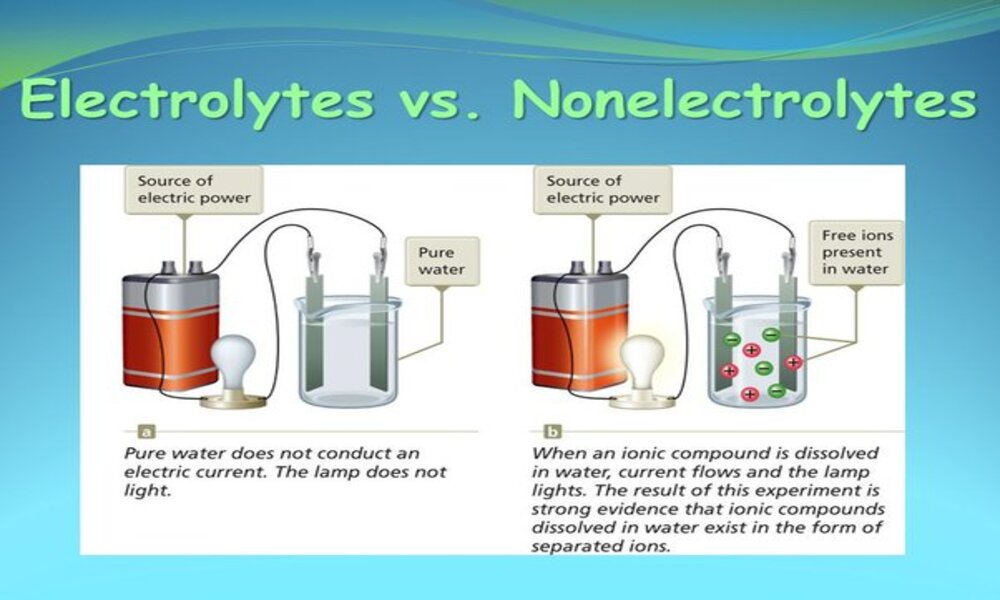
How Do Electrolytes And Nonelectrolytes Differ . Electrolytes are chemicals that break into ions (ionize) when. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Nonelectrolytes, on the other hand, maintain their molecular.
from keydifference.in
Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. Nonelectrolytes, on the other hand, maintain their molecular. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. Electrolytes are chemicals that break into ions (ionize) when.
Top 17Difference Between Electrolytes And Nonelectrolytes Key Difference
How Do Electrolytes And Nonelectrolytes Differ Nonelectrolytes, on the other hand, maintain their molecular. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Nonelectrolytes, on the other hand, maintain their molecular. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes are chemicals that break into ions (ionize) when.
From www.youtube.com
Identifying electrolytes vs. nonelectrolytes based on particle diagrams YouTube How Do Electrolytes And Nonelectrolytes Differ When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes are chemicals that break into ions (ionize). How Do Electrolytes And Nonelectrolytes Differ.
From slideplayer.com
Chapter 3 Chemical Compounds. ppt download How Do Electrolytes And Nonelectrolytes Differ When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes are chemicals that break into ions (ionize) when. Nonelectrolytes, on the other hand, maintain their molecular. Electrolytes and nonelectrolytes. How Do Electrolytes And Nonelectrolytes Differ.
From www.youtube.com
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes Chemistry Examples How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Nonelectrolytes, on the other hand, maintain their molecular. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideserve.com
PPT Solutions Review Pt 2 PowerPoint Presentation, free download ID2220376 How Do Electrolytes And Nonelectrolytes Differ The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. Nonelectrolytes, on the other hand, maintain their molecular. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Electrolytes are chemicals that break into ions (ionize) when. An electrolyte is a compound that conducts. How Do Electrolytes And Nonelectrolytes Differ.
From melissa-media.blogspot.com
Melissa Media Strong And Weak Electrolytes How Do Electrolytes And Nonelectrolytes Differ Nonelectrolytes, on the other hand, maintain their molecular. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. An. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideserve.com
PPT Solutions Unit PowerPoint Presentation, free download ID1980079 How Do Electrolytes And Nonelectrolytes Differ The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Nonelectrolytes, on the other hand, maintain their molecular. Electrolytes are chemicals that break into ions (ionize) when. When some substances are dissolved in water,. How Do Electrolytes And Nonelectrolytes Differ.
From www.numerade.com
SOLVED Questions and Problems Electrolytes and Nonelectrolytes 17. KF is a strong electrolyte How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. Nonelectrolytes, on the other hand, maintain their molecular. When electrolytes dissolve, they release positive and negative ions, which are responsible for. How Do Electrolytes And Nonelectrolytes Differ.
From keydifference.in
Top 17Difference Between Electrolytes And Nonelectrolytes Key Difference How Do Electrolytes And Nonelectrolytes Differ Nonelectrolytes, on the other hand, maintain their molecular. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in. How Do Electrolytes And Nonelectrolytes Differ.
From pediaa.com
Difference Between Strong and Weak Electrolytes Definition, Properties, Reactions How Do Electrolytes And Nonelectrolytes Differ Nonelectrolytes, on the other hand, maintain their molecular. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. Electrolytes are chemicals that break into ions (ionize) when. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. When electrolytes dissolve, they release positive and negative. How Do Electrolytes And Nonelectrolytes Differ.
From www.pinterest.com
Electrolytes vs Nonelectrolytes Secondary science, Brain book, Chemistry How Do Electrolytes And Nonelectrolytes Differ Nonelectrolytes, on the other hand, maintain their molecular. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but. How Do Electrolytes And Nonelectrolytes Differ.
From www.majordifferences.com
Strong Electrolytes vs Weak Electrolytes Major Differences How Do Electrolytes And Nonelectrolytes Differ When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Nonelectrolytes, on the other hand, maintain their molecular. Electrolytes are chemicals that break into ions (ionize) when. Electrolytes and nonelectrolytes are distinct types of substances. How Do Electrolytes And Nonelectrolytes Differ.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL How Do Electrolytes And Nonelectrolytes Differ Electrolytes are chemicals that break into ions (ionize) when. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. Nonelectrolytes, on the other hand, maintain their molecular. When some substances are. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideserve.com
PPT More on solutions… PowerPoint Presentation, free download ID6075986 How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Nonelectrolytes, on the other hand, maintain their molecular. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When electrolytes dissolve, they release positive and negative ions, which are responsible. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry PowerPoint Presentation ID6291419 How Do Electrolytes And Nonelectrolytes Differ When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Electrolytes are chemicals that break into ions (ionize) when. The main difference between the two is that electrolytes can conduct electricity when dissolved. How Do Electrolytes And Nonelectrolytes Differ.
From ar.inspiredpencil.com
Electrolytes And Nonelectrolytes How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. The main difference between the two. How Do Electrolytes And Nonelectrolytes Differ.
From www.numerade.com
SOLVED QUESTION 24 How does an electrolyte differ from nonelectrolyte? Electrolytes are less How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Nonelectrolytes, on the other hand, maintain their molecular. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. Electrolytes are chemicals that break into ions (ionize) when. An electrolyte is a compound that conducts an electric. How Do Electrolytes And Nonelectrolytes Differ.
From pediaa.com
Difference Between Electrolytes and Nonelectrolytes Definition, Properties, Examples How Do Electrolytes And Nonelectrolytes Differ When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Electrolytes are chemicals that break into ions (ionize) when. Nonelectrolytes, on the other hand, maintain their molecular. Electrolytes and nonelectrolytes are distinct types. How Do Electrolytes And Nonelectrolytes Differ.
From www.shutterstock.com
19 Strong Electrolyte And Weak Electrolyte Images, Stock Photos & Vectors Shutterstock How Do Electrolytes And Nonelectrolytes Differ The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. Nonelectrolytes, on the other hand, maintain their molecular. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When electrolytes dissolve, they release positive and negative ions, which are responsible for. How Do Electrolytes And Nonelectrolytes Differ.
From www.askdifference.com
Electrolytes vs. Nonelectrolytes — What’s the Difference? How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes are chemicals that break into ions (ionize) when. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes and nonelectrolytes are distinct types of substances with different attributes. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideserve.com
PPT Chemical reactions PowerPoint Presentation, free download ID6891063 How Do Electrolytes And Nonelectrolytes Differ When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes and nonelectrolytes are distinct types. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID2437162 How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. An electrolyte is a. How Do Electrolytes And Nonelectrolytes Differ.
From thecontentauthority.com
Electrolytes vs Nonelectrolytes Meaning And Differences How Do Electrolytes And Nonelectrolytes Differ Electrolytes are chemicals that break into ions (ionize) when. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes and nonelectrolytes are distinct types of substances with different attributes. How Do Electrolytes And Nonelectrolytes Differ.
From www.youtube.com
Identifying an electrolyte vs. a nonelectrolyte YouTube How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. Nonelectrolytes, on the other hand, maintain their molecular. When some. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solutions PowerPoint Presentation ID6778248 How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Nonelectrolytes, on the other. How Do Electrolytes And Nonelectrolytes Differ.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps How Do Electrolytes And Nonelectrolytes Differ Nonelectrolytes, on the other hand, maintain their molecular. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. An electrolyte is a compound that conducts an electric current when. How Do Electrolytes And Nonelectrolytes Differ.
From www.youtube.com
The Difference between Weak Electrolytes, Strong Electrolytes, and Nonelectrolytes YouTube How Do Electrolytes And Nonelectrolytes Differ The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. Nonelectrolytes, on the other hand, maintain their molecular. An. How Do Electrolytes And Nonelectrolytes Differ.
From www.pathwaystochemistry.com
Electrolytes and Nonelectrolytes Pathways to Chemistry How Do Electrolytes And Nonelectrolytes Differ When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Nonelectrolytes, on the other hand, maintain their molecular. When electrolytes dissolve, they release positive and negative ions, which are responsible. How Do Electrolytes And Nonelectrolytes Differ.
From www.scribd.com
7 2 Electrolytes and Nonelectrolytes PDF Electrolyte Dissociation (Chemistry) How Do Electrolytes And Nonelectrolytes Differ When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes are chemicals that break into ions (ionize) when. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. An electrolyte is a compound that conducts an electric current when it is in. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideserve.com
PPT Warm Up 7.1 Review of Naming & Bonding PowerPoint Presentation ID2954486 How Do Electrolytes And Nonelectrolytes Differ Nonelectrolytes, on the other hand, maintain their molecular. Electrolytes are chemicals that break into ions (ionize) when. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. When some substances are dissolved in water, they. How Do Electrolytes And Nonelectrolytes Differ.
From www.difference.wiki
Electrolytes vs. Nonelectrolytes What’s the Difference? How Do Electrolytes And Nonelectrolytes Differ Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. When electrolytes dissolve, they release positive and negative ions, which are responsible for conducting electric current. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. The main difference between the two is that electrolytes can. How Do Electrolytes And Nonelectrolytes Differ.
From www.phartoonz.com
List of electrolytes and their normal range Phartoonz How Do Electrolytes And Nonelectrolytes Differ The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution.. How Do Electrolytes And Nonelectrolytes Differ.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes How Do Electrolytes And Nonelectrolytes Differ An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. The main difference between the two is that electrolytes can conduct electricity when dissolved in water, but nonelectrolytes can not. Electrolytes are chemicals that break into. How Do Electrolytes And Nonelectrolytes Differ.
From www.slideshare.net
electrolytes and nonelectrolytes How Do Electrolytes And Nonelectrolytes Differ When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Nonelectrolytes, on the other hand,. How Do Electrolytes And Nonelectrolytes Differ.
From www.scribd.com
2 Electrolytes and Nonelectrolytes PDF How Do Electrolytes And Nonelectrolytes Differ Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When electrolytes dissolve, they release. How Do Electrolytes And Nonelectrolytes Differ.
From www.youtube.com
Electrolytes and nonelectrolytes / best chemistry solution YouTube How Do Electrolytes And Nonelectrolytes Differ Electrolytes and nonelectrolytes are distinct types of substances with different attributes and behaviors in solutions. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes are chemicals that break into ions. How Do Electrolytes And Nonelectrolytes Differ.