Differential Adsorption Meaning . the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat.
from www.theengineersperspectives.com
adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat.
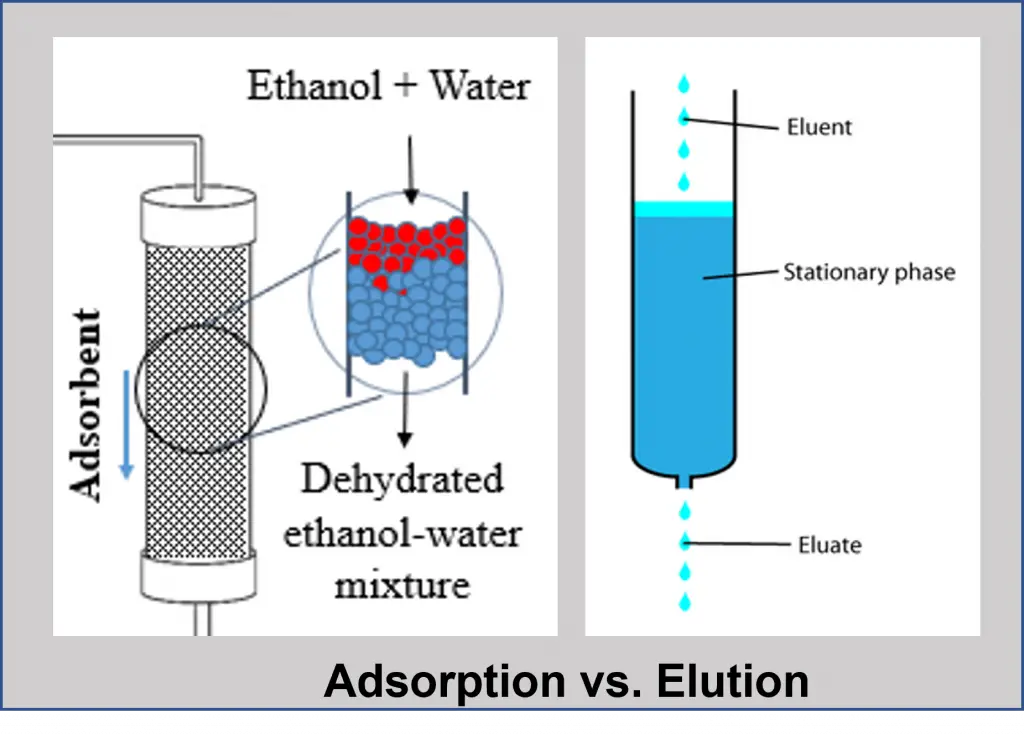
Elution vs. Adsorption The Engineer's Perspective
Differential Adsorption Meaning the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition.
From www.slideserve.com
PPT Adsorption/Partition Chromatography PowerPoint Presentation, free download ID9650462 Differential Adsorption Meaning adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. The mixture of gas. Differential Adsorption Meaning.
From www.theengineeringconcepts.com
Absorption Vs Adsorption The Engineering Concepts Differential Adsorption Meaning the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at. Differential Adsorption Meaning.
From www.slideserve.com
PPT ADSORPTION PowerPoint Presentation, free download ID1757925 Differential Adsorption Meaning this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. heat of adsorption is. Differential Adsorption Meaning.
From www.researchgate.net
Adsorption structure and charge differential density diagram of N2... Download Scientific Diagram Differential Adsorption Meaning the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. . Differential Adsorption Meaning.
From www.researchgate.net
(a) Differential adsorption Gibbs free energies for Pt(221) with the... Download Scientific Differential Adsorption Meaning heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. . Differential Adsorption Meaning.
From www.researchgate.net
Adsorption structure and charge differential density diagram of N2... Download Scientific Diagram Differential Adsorption Meaning this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. the. Differential Adsorption Meaning.
From www.youtube.com
Mechanism of Adsorption NCERT Explained Surface chemistry Class 12th YouTube Differential Adsorption Meaning the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the. Differential Adsorption Meaning.
From www.researchgate.net
Figure S30. Loadingdependent differential enthalpy of H2O adsorption... Download Scientific Differential Adsorption Meaning differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. this review paper focuses. Differential Adsorption Meaning.
From www.researchgate.net
Differential heat of adsorption of CP at 303.15 K on GBACG70R and... Download Scientific Diagram Differential Adsorption Meaning adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while.. Differential Adsorption Meaning.
From www.theengineersperspectives.com
Elution vs. Adsorption The Engineer's Perspective Differential Adsorption Meaning differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. the. Differential Adsorption Meaning.
From www.researchgate.net
14. The differential heat of adsorption q d (N )/k B at T = 60, 75, and... Download Scientific Differential Adsorption Meaning heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. The mixture of gas or. Differential Adsorption Meaning.
From www.researchgate.net
(a) Differential doublelayer capacitance (C dl ), adsorption... Download Scientific Diagram Differential Adsorption Meaning this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. The mixture of gas or. Differential Adsorption Meaning.
From www.researchgate.net
Figure S1. Coverage dependence of differential adsorption energy of the... Download Scientific Differential Adsorption Meaning The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. heat. Differential Adsorption Meaning.
From www.researchgate.net
Adsorption isotherms (A), differential adsorption potential... Download Scientific Diagram Differential Adsorption Meaning adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. . Differential Adsorption Meaning.
From nmk.world
Absorption vs Adsorption Key Differences and Examples Differential Adsorption Meaning differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. adsorption chromatography involves the analytical separation of. Differential Adsorption Meaning.
From www.researchgate.net
Differential adsorption enthalpy of CO 2 on CaO(001)/Pt(001) measured... Download Scientific Differential Adsorption Meaning the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. The. Differential Adsorption Meaning.
From www.researchgate.net
Differential heats of adsorption, as a function of CO adsorbed amounts,... Download Scientific Differential Adsorption Meaning The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. the. Differential Adsorption Meaning.
From www.differencebetween.com
Difference Between Adsorption and Desorption Compare the Difference Between Similar Terms Differential Adsorption Meaning adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. differential. Differential Adsorption Meaning.
From www.slideserve.com
PPT Adsorption (part 1) PowerPoint Presentation, free download ID4621071 Differential Adsorption Meaning The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects. Differential Adsorption Meaning.
From www.slideshare.net
Adsorption Differential Adsorption Meaning The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects. Differential Adsorption Meaning.
From www.researchgate.net
Strategy for differential adsorption of flexible guests. a) Controlling... Download Scientific Differential Adsorption Meaning heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. this review paper. Differential Adsorption Meaning.
From www.researchgate.net
Differential heat of adsorption of ethanol on the four catalysts Download Scientific Diagram Differential Adsorption Meaning The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. . Differential Adsorption Meaning.
From www.youtube.com
Diff betn Physisorption and chemisorption,Definition of Extent of Adsorption,Ads isotherm,Ads Differential Adsorption Meaning the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption. Differential Adsorption Meaning.
From www.researchgate.net
(a) Differential adsorption Gibbs free energies for Pt(211). The two... Download Scientific Differential Adsorption Meaning differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. adsorption chromatography involves the. Differential Adsorption Meaning.
From www.researchgate.net
Differential heat of adsorption of acetaldehyde on Co/ZnO and Co/ZnO(d)... Download Scientific Differential Adsorption Meaning the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. adsorption. Differential Adsorption Meaning.
From www.researchgate.net
(a) General mechanism for the adsorption, (b) monolayer adsorption, and... Download Scientific Differential Adsorption Meaning this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the. Differential Adsorption Meaning.
From studiousguy.com
8 Examples of Adsorption in Daily Life StudiousGuy Differential Adsorption Meaning differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the. Differential Adsorption Meaning.
From www.researchgate.net
Adsorption calculated as the differential of mass change with... Download Scientific Differential Adsorption Meaning The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects. Differential Adsorption Meaning.
From www.researchgate.net
Concept of differential protein adsorption the physicochemical... Download Scientific Diagram Differential Adsorption Meaning adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. adsorption chromatography involves the analytical separation. Differential Adsorption Meaning.
From sciencenotes.org
Adsorption vs Absorption Differences and Examples Differential Adsorption Meaning the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with. Differential Adsorption Meaning.
From www.researchgate.net
Calculated differential adsorption energy of hydrogen as a function of... Download Scientific Differential Adsorption Meaning adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. this review paper focuses on the formation and adsorption of clay minerals and their influence on organic. the primary difference between adsorption and absorption is that adsorption occurs when particles adhere to the surface of a substance, while. The mixture of gas. Differential Adsorption Meaning.
From www.researchgate.net
(a) Differential adsorption Gibbs free energies for Pt(221) with the... Download Scientific Differential Adsorption Meaning adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. the. Differential Adsorption Meaning.
From www.youtube.com
Adsorption Vs Absorption (Differences) YouTube Differential Adsorption Meaning adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. the primary difference between. Differential Adsorption Meaning.
From pt.slideshare.net
Adsorption Differential Adsorption Meaning adsorption chromatography involves the analytical separation of a chemical mixture based on the interaction of the adsorbate with the adsorbent. differential or integral entropies of adsorption can be obtained from calorimetry and together with an adsorption isotherm,. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different. Differential Adsorption Meaning.
From www.researchgate.net
(a) Differential adsorption Gibbs free energies for Pt(211). The two... Download Scientific Differential Adsorption Meaning heat of adsorption is a basic thermodynamic property extensively used not only for understanding thermal effects and heat. The mixture of gas or liquid gets separated when it passes over the adsorbent bed that adsorbs different compounds at different rates. adsorption chromatography involves the selective adsorption of components onto a solid stationary phase, while partition. differential or. Differential Adsorption Meaning.