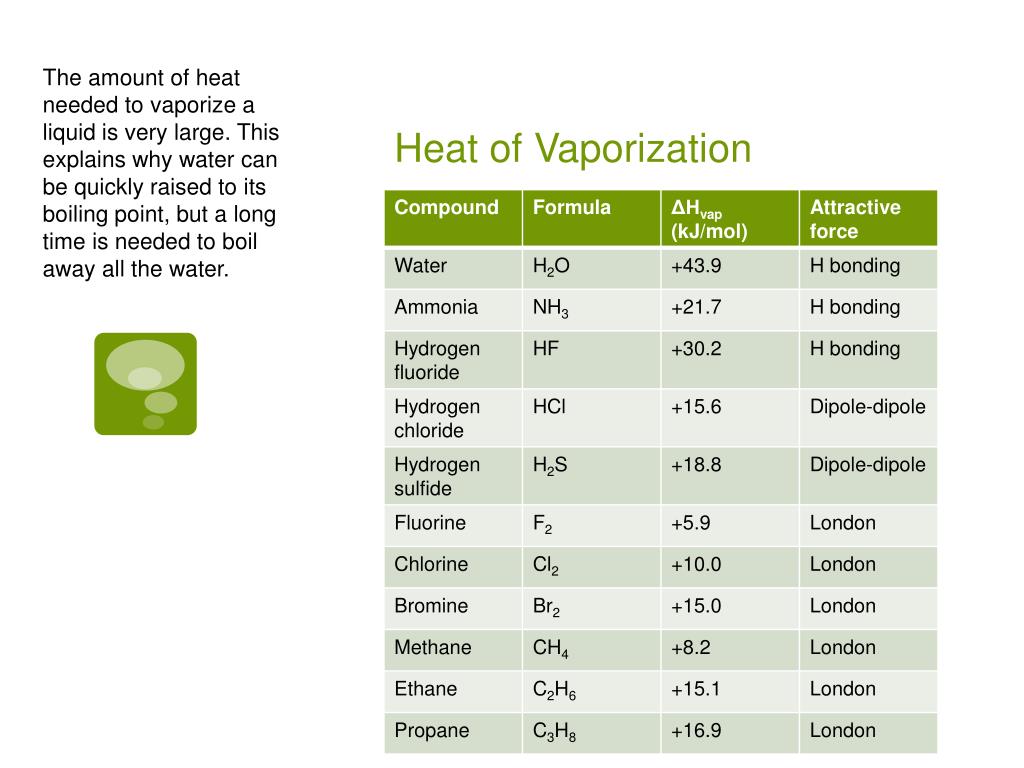
Has The Highest Heat Of Vaporization . Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. L f l f and l v l v are. Much more energy is required to. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Water’s cohesive forces allow for the property of surface tension,. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Water has a heat of vaporization value of 40.65 kj/mol. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat.
from www.slideserve.com
The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. Water has a heat of vaporization value of 40.65 kj/mol. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. L f l f and l v l v are. Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. Water’s cohesive forces allow for the property of surface tension,. Much more energy is required to. The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure.
PPT Liquids and Solids PowerPoint Presentation, free download ID
Has The Highest Heat Of Vaporization Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Water has a heat of vaporization value of 40.65 kj/mol. L f l f and l v l v are. Much more energy is required to. Water’s cohesive forces allow for the property of surface tension,. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization.
From www.chegg.com
Solved Which has the highest heat of vaporization? SbH3 Has The Highest Heat Of Vaporization Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Has The Highest Heat Of Vaporization L f l f and l v l v are. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Notice that. Has The Highest Heat Of Vaporization.
From slidecourse.blogspot.com
Latent Heat Of Vaporization Equation Slide Course Has The Highest Heat Of Vaporization The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. Water has a heat of vaporization value of 40.65 kj/mol. Water also exhibits a high heat of vaporization, which is key to how. Has The Highest Heat Of Vaporization.
From education-portal.com
Heat of Vaporization Definition & Equation Video & Lesson Transcript Has The Highest Heat Of Vaporization Water’s cohesive forces allow for the property of surface tension,. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at. Has The Highest Heat Of Vaporization.
From www.ck12.org
Heats of Vaporization and Condensation CK12 Foundation Has The Highest Heat Of Vaporization The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. Much more energy is required to. Water has a heat of vaporization value of 40.65 kj/mol. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at. Has The Highest Heat Of Vaporization.
From www.slideshare.net
Enthalpy of vaporization of liquid Has The Highest Heat Of Vaporization Water has a heat of vaporization value of 40.65 kj/mol. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Water’s cohesive forces allow for the property of surface tension,. L f l f and l v l v are. The heat of. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID4252232 Has The Highest Heat Of Vaporization Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. Water has a heat of vaporization value of 40.65 kj/mol. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Water also exhibits a high heat. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID5578663 Has The Highest Heat Of Vaporization As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. The. Has The Highest Heat Of Vaporization.
From www.numerade.com
SOLVED Which is expected to have the highest heat of vaporization? A Has The Highest Heat Of Vaporization Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Water has a heat of vaporization value of 40.65 kj/mol. Much more energy is required to.. Has The Highest Heat Of Vaporization.
From www.numerade.com
SOLVED NH3, NHF2, NF3 which has the highest enthalpy of vaporization Has The Highest Heat Of Vaporization As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Do Now PowerPoint Presentation, free download ID4716111 Has The Highest Heat Of Vaporization Much more energy is required to. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. L f l f and l v l v are. The heat of vaporization is the. Has The Highest Heat Of Vaporization.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Has The Highest Heat Of Vaporization The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. Much more energy is required to. Water’s cohesive forces allow for the property of surface tension,.. Has The Highest Heat Of Vaporization.
From engineerexcel.com
Heat of Vaporization Explained EngineerExcel Has The Highest Heat Of Vaporization The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. Much more energy is required to. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. Water’s cohesive forces allow for the property of surface tension,. Water has a heat of vaporization value. Has The Highest Heat Of Vaporization.
From www.showme.com
Heat of vaporization Science, Chemistry ShowMe Has The Highest Heat Of Vaporization The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. Water has a heat of vaporization value of 40.65 kj/mol. L f l f and. Has The Highest Heat Of Vaporization.
From www.ck12.org
Heat of vaporization Example 1 ( Video ) Chemistry CK12 Foundation Has The Highest Heat Of Vaporization The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Water’s cohesive forces allow for the property of surface tension,. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Has The Highest Heat Of Vaporization L f l f and l v l v are. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. Water has a heat of vaporization. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download Has The Highest Heat Of Vaporization L f l f and l v l v are. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. The heat (or enthalpy) of vaporization is the quantity of heat that. Has The Highest Heat Of Vaporization.
From www.numerade.com
VIDEO solution Which of the following substances is expected to have Has The Highest Heat Of Vaporization The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. L f l f and l v l v are. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor,. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Atmospheric Moisture PowerPoint Presentation, free download ID Has The Highest Heat Of Vaporization Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. Water’s cohesive forces allow for the property of surface tension,. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. Notice that for all substances, the heat of vaporization. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Temperature and Heat PowerPoint Presentation, free download ID Has The Highest Heat Of Vaporization The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Much more energy is required to. Water’s cohesive forces allow for the property of surface tension,. Water has a heat of vaporization value of 40.65 kj/mol. Notice that for all substances, the. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 Has The Highest Heat Of Vaporization Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. Water’s cohesive forces allow for the property of surface tension,. Much more energy is required to. L f l f and l v l v are. Water has a heat of vaporization value of 40.65 kj/mol. The heat (or. Has The Highest Heat Of Vaporization.
From heatklp.blogspot.com
11+ Why Is Heat Of Vaporization Important To Life On Earth Article Has The Highest Heat Of Vaporization Water’s cohesive forces allow for the property of surface tension,. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. The heat of. Has The Highest Heat Of Vaporization.
From engineerexcel.com
Heat of Vaporization Explained EngineerExcel Has The Highest Heat Of Vaporization Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. Water has a heat of vaporization value of 40.65 kj/mol. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. The latent heat of vaporization is the amount of heat needed to. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID5769943 Has The Highest Heat Of Vaporization As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas. Has The Highest Heat Of Vaporization.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo Has The Highest Heat Of Vaporization Much more energy is required to. Water’s cohesive forces allow for the property of surface tension,. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion.. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Chapter 12 Liquids and Solids PowerPoint Presentation, free Has The Highest Heat Of Vaporization The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Water also exhibits a high. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT State Changes PowerPoint Presentation, free download ID6588117 Has The Highest Heat Of Vaporization The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. Much more energy is required to. Water has a heat of vaporization. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Ch. 11 Liquids, Solids, and Intermolecular Forces PowerPoint Has The Highest Heat Of Vaporization Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. As a result of the network of hydrogen bonding present between water molecules, a high input. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Water Chemistry & Properties of Water PowerPoint Presentation Has The Highest Heat Of Vaporization As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. Water also exhibits a high heat of vaporization, which is key to how organisms cool themselves by the evaporation of sweat. Much. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT The Extraordinary Properties of Water PowerPoint Presentation Has The Highest Heat Of Vaporization The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Water has a heat of vaporization value of 40.65 kj/mol. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. As a. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download Has The Highest Heat Of Vaporization L f l f and l v l v are. The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Water’s cohesive forces allow for the property of surface tension,. Much more energy is required to. Notice that for all substances, the. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Latent Heat PowerPoint Presentation ID5759776 Has The Highest Heat Of Vaporization Much more energy is required to. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. As a result of the network of. Has The Highest Heat Of Vaporization.
From www.sliderbase.com
Adhesion Has The Highest Heat Of Vaporization Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. Water’s cohesive forces allow for. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT b) substances also are important to organisms, but do Has The Highest Heat Of Vaporization The heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and pressure. Water’s cohesive forces allow for the property of surface tension,. The latent heat of vaporization is the amount of heat needed to cause a phase change between liquid and gas. Water has. Has The Highest Heat Of Vaporization.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID5769198 Has The Highest Heat Of Vaporization Much more energy is required to. The heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature. Notice that for all substances, the heat of vaporization is substantially higher than the heat of fusion. As a result of the network of hydrogen bonding present. Has The Highest Heat Of Vaporization.