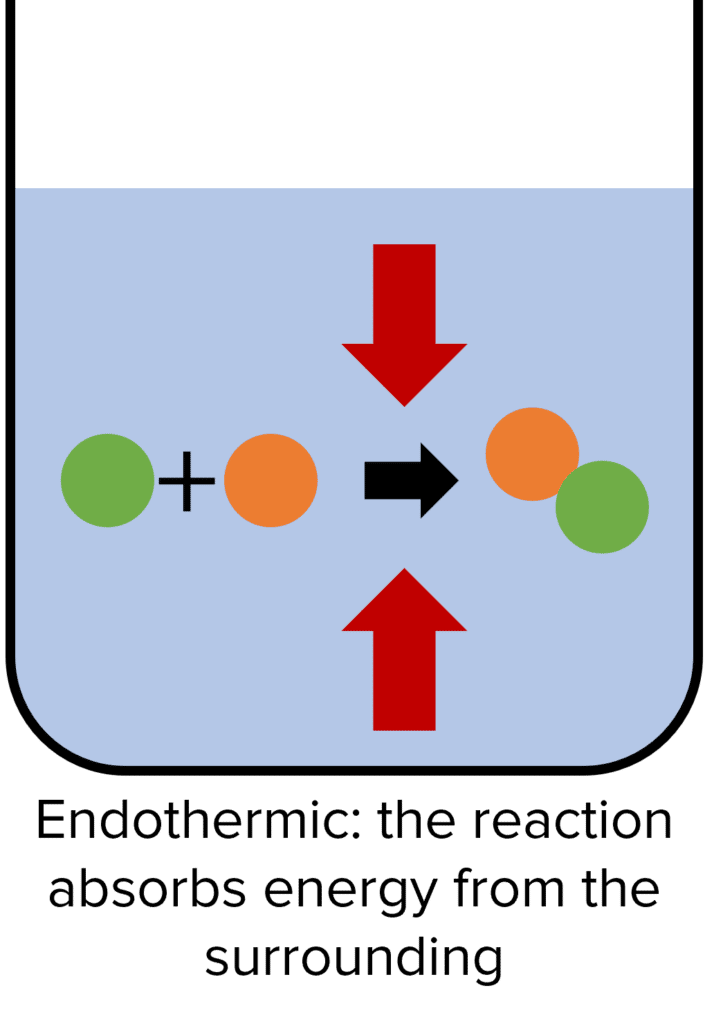
Endothermic Reaction Temperature . in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. some examples of endothermic reactions are: when temperature is the stress that affects a system at equilibrium, there are two important consequences: The reaction between ethanoic acid and sodium carbonate. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.
from mmerevise.co.uk
some examples of endothermic reactions are: in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. The reaction between ethanoic acid and sodium carbonate. when temperature is the stress that affects a system at equilibrium, there are two important consequences: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings.
Endothermic and Exothermic Reactions Revision MME
Endothermic Reaction Temperature The reaction between ethanoic acid and sodium carbonate. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. The reaction between ethanoic acid and sodium carbonate. some examples of endothermic reactions are: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. when temperature is the stress that affects a system at equilibrium, there are two important consequences:
From classzonesalicetum.z14.web.core.windows.net
Identify Endothermic And Exothermic Reactions Endothermic Reaction Temperature when temperature is the stress that affects a system at equilibrium, there are two important consequences: The reaction between ethanoic acid and sodium carbonate. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to. Endothermic Reaction Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. when temperature is the stress that affects a system at equilibrium, there are two important consequences: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. some examples of endothermic reactions are:. Endothermic Reaction Temperature.
From slidetodoc.com
Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1 Endothermic Reaction Temperature — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. when temperature is the stress that affects a system at equilibrium, there are two important consequences: The reaction between ethanoic acid and sodium carbonate. in the course of an endothermic process, the system gains heat from the surroundings and so the. Endothermic Reaction Temperature.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Temperature when temperature is the stress that affects a system at equilibrium, there are two important consequences: The reaction between ethanoic acid and sodium carbonate. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in the course. Endothermic Reaction Temperature.
From www.expii.com
Endothermic and Exothermic Reactions — Overview & Comparison Expii Endothermic Reaction Temperature — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. when temperature is the stress that affects a system at equilibrium, there are two important consequences: These reactions lower the temperature of. Endothermic Reaction Temperature.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram Endothermic Reaction Temperature some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. in the course of an endothermic process, the system gains heat from the. Endothermic Reaction Temperature.
From treatybottle13.pythonanywhere.com
Fantastic 10 Examples Of Endothermic Reactions With Equations Edexcel Igcse Further Pure Endothermic Reaction Temperature in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The reaction between ethanoic acid and sodium carbonate. when temperature is the stress that affects a system at equilibrium, there are two important. Endothermic Reaction Temperature.
From www.preproom.org
Exothermic and Endothermic Reactions Science Practical Expiriment used in School and Education Endothermic Reaction Temperature when temperature is the stress that affects a system at equilibrium, there are two important consequences: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. some examples of endothermic reactions are: — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. —. Endothermic Reaction Temperature.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Reaction Temperature in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. when temperature is the stress that affects a system at equilibrium, there are two important consequences: some examples of. Endothermic Reaction Temperature.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Temperature — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. when temperature is the stress that affects a system at equilibrium, there are two important consequences: — endothermic reactions. Endothermic Reaction Temperature.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Temperature in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. The reaction between ethanoic acid and sodium carbonate. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. some examples of endothermic reactions are: These reactions lower the temperature of. Endothermic Reaction Temperature.
From studyfinder.org
Understanding the Difference Endothermic Reaction vs Exothermic Reaction Worksheet Answer Key Endothermic Reaction Temperature when temperature is the stress that affects a system at equilibrium, there are two important consequences: — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. The reaction between ethanoic acid and. Endothermic Reaction Temperature.
From stock.adobe.com
endothermic reaction graph in chemistry Stock Vector Adobe Stock Endothermic Reaction Temperature — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. . Endothermic Reaction Temperature.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Temperature — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. when temperature is the stress that affects a system at equilibrium, there are two important consequences: These. Endothermic Reaction Temperature.
From www.slideserve.com
PPT Exothermic and Endothermic Reactions PowerPoint Presentation, free download ID4499042 Endothermic Reaction Temperature some examples of endothermic reactions are: — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. The reaction. Endothermic Reaction Temperature.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction Temperature in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. when temperature is the stress that affects a system at equilibrium, there are two important consequences: — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These. Endothermic Reaction Temperature.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Temperature — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The reaction between ethanoic acid and sodium carbonate. when temperature is the stress that affects a system at equilibrium, there are two important consequences: some examples of endothermic reactions are: These reactions lower the temperature of their surrounding. Endothermic Reaction Temperature.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Reaction Temperature The reaction between ethanoic acid and sodium carbonate. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. when temperature is the stress that affects a system at equilibrium, there are two important consequences: in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the. Endothermic Reaction Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. when temperature is the stress that affects a system at equilibrium, there are two important consequences: in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. some examples of endothermic reactions are:. Endothermic Reaction Temperature.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Resources Endothermic Reaction Temperature when temperature is the stress that affects a system at equilibrium, there are two important consequences: — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. in the course of an endothermic process, the. Endothermic Reaction Temperature.
From diagramdataconfusion.z22.web.core.windows.net
Endothermic Energy Diagram Endothermic Reaction Temperature when temperature is the stress that affects a system at equilibrium, there are two important consequences: — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. . Endothermic Reaction Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. . Endothermic Reaction Temperature.
From nerdyseal.com
Temperature and endothermic reaction 387 Words NerdySeal Endothermic Reaction Temperature The reaction between ethanoic acid and sodium carbonate. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. — an endothermic reaction occurs when the temperature of. Endothermic Reaction Temperature.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Temperature — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. The. Endothermic Reaction Temperature.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation, free download ID5913704 Endothermic Reaction Temperature These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. some examples of endothermic reactions are: — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. when. Endothermic Reaction Temperature.
From www.slideserve.com
PPT Heat from Reactions PowerPoint Presentation, free download ID3481076 Endothermic Reaction Temperature These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. some examples of endothermic reactions are:. Endothermic Reaction Temperature.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Temperature — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. when temperature is the stress that affects a system at equilibrium, there are two important consequences: The reaction between ethanoic acid and sodium carbonate. in the course of an endothermic process, the system gains heat from the surroundings. Endothermic Reaction Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature some examples of endothermic reactions are: — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings.. Endothermic Reaction Temperature.
From manuallistbanderole.z13.web.core.windows.net
Energy Level Diagram For Endothermic Reaction Endothermic Reaction Temperature in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. when temperature is the stress that affects a system at equilibrium, there are two important consequences: some examples of endothermic reactions are:. Endothermic Reaction Temperature.
From www.pinterest.it
Endothermic and Exothermic Reactions Lab ⋆ Exothermic reaction, Teaching Endothermic Reaction Temperature some examples of endothermic reactions are: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. when temperature is the stress that affects a system at equilibrium, there are two important consequences: in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings.. Endothermic Reaction Temperature.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Endothermic Reaction Temperature when temperature is the stress that affects a system at equilibrium, there are two important consequences: in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The. Endothermic Reaction Temperature.
From general.chemistrysteps.com
What is Enthalpy Chemistry Steps Endothermic Reaction Temperature — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. when temperature is the stress that affects a system at equilibrium, there are two important consequences: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The reaction between ethanoic acid and sodium carbonate. . Endothermic Reaction Temperature.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation, free download ID5913704 Endothermic Reaction Temperature These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. when temperature is the stress that affects a system at equilibrium, there are two important consequences: — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. — endothermic reactions are chemical reactions in which the reactants absorb. Endothermic Reaction Temperature.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Endothermic Reaction Temperature These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. when temperature is the stress that affects a system at equilibrium, there are two important consequences: — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The reaction between ethanoic acid and sodium carbonate. . Endothermic Reaction Temperature.
From www.numerade.com
SOLVED Which one of the following given graphs represents the variation of rate constant (k Endothermic Reaction Temperature The reaction between ethanoic acid and sodium carbonate. — an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. — endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. when temperature is the stress that affects a system at equilibrium, there are two. Endothermic Reaction Temperature.