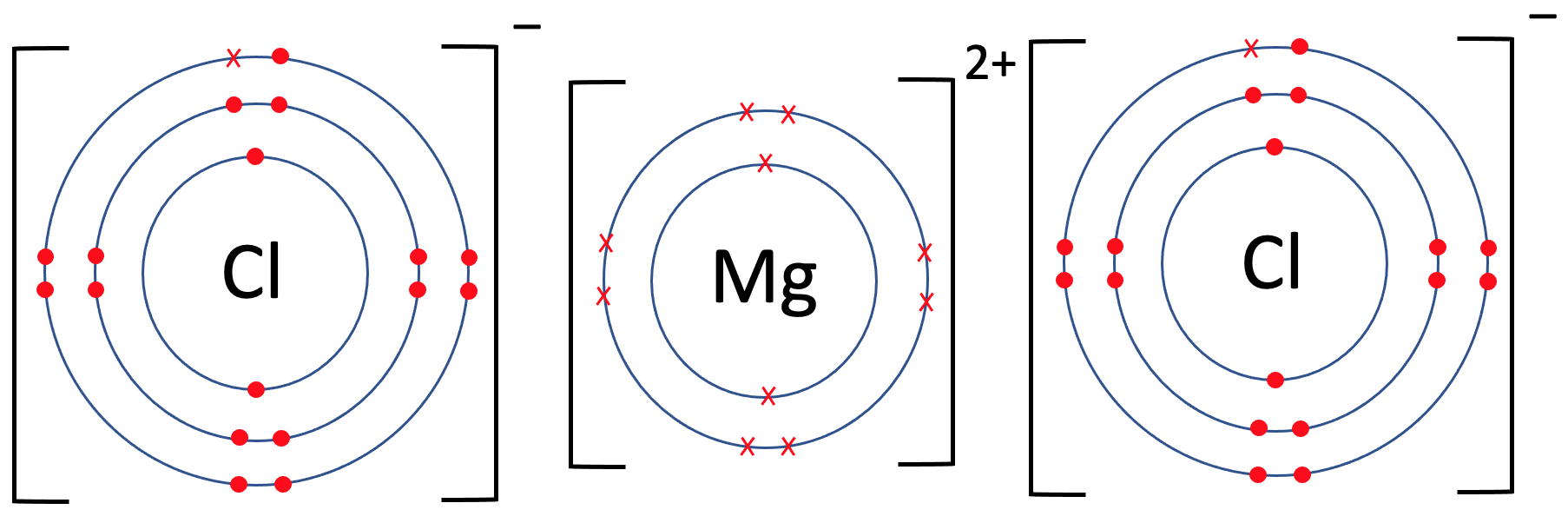
Chloride Ion In Magnesium . Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. It is an ionic compound, composed. Thus, the ratio of magnesium ion: 2 and the simplest magnesium. So, magnesium ions form the ionic bond with two chlorine ions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Now consider the ionic compound formed by magnesium and chlorine. This is two positive charges and two negative charges. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:
from www.elevise.co.uk
2 and the simplest magnesium. Thus, the ratio of magnesium ion: This is two positive charges and two negative charges. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Now consider the ionic compound formed by magnesium and chlorine. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. So, magnesium ions form the ionic bond with two chlorine ions. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. It is an ionic compound, composed.
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise
Chloride Ion In Magnesium So, magnesium ions form the ionic bond with two chlorine ions. So, magnesium ions form the ionic bond with two chlorine ions. Now consider the ionic compound formed by magnesium and chlorine. Thus, the ratio of magnesium ion: This is two positive charges and two negative charges. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. 2 and the simplest magnesium. It is an ionic compound, composed. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride.
From brainly.in
Show the formation of magnesium chloride Brainly.in Chloride Ion In Magnesium The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. 2 and the simplest magnesium. Thus, the ratio. Chloride Ion In Magnesium.
From www.vrogue.co
Gcse Chemistry Aqa 9 1 Ionic Bonding Dot And Cross Di vrogue.co Chloride Ion In Magnesium Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. 2 and the simplest magnesium. So, magnesium ions form the ionic bond with two chlorine ions. At the heart of magnesium chloride’s usefulness is its unique set. Chloride Ion In Magnesium.
From slideplayer.com
Quantitative Chemistry ppt download Chloride Ion In Magnesium So, magnesium ions form the ionic bond with two chlorine ions. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Now consider the ionic compound formed by magnesium and chlorine. A magnesium ion has a. Chloride Ion In Magnesium.
From www.numerade.com
SOLVED Magnesium and chlorine can be made by the electrolysis of Chloride Ion In Magnesium About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. It is an ionic compound, composed. Now consider the ionic compound formed by magnesium and chlorine. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium. Chloride Ion In Magnesium.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Chloride Ion In Magnesium Thus, the ratio of magnesium ion: So, magnesium ions form the ionic bond with two chlorine ions. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride,. Chloride Ion In Magnesium.
From brainly.in
Write the formation of magnesium chloride (MgCl,) with the help of Chloride Ion In Magnesium It is an ionic compound, composed. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 2 and the simplest magnesium. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium. Chloride Ion In Magnesium.
From www.slideserve.com
PPT CHAPTER 3 METALS AND NON METALS PowerPoint Presentation ID598959 Chloride Ion In Magnesium It is an ionic compound, composed. So, magnesium ions form the ionic bond with two chlorine ions. Thus, the ratio of magnesium ion: The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. 2 and the simplest. Chloride Ion In Magnesium.
From www.toppr.com
Draw electron dot representation for the formation of magnesium chloride. Chloride Ion In Magnesium Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: It is an ionic compound, composed. So, magnesium ions form the ionic bond with. Chloride Ion In Magnesium.
From www.edplace.com
Analyse Ionic bonding Worksheet EdPlace Chloride Ion In Magnesium Thus, the ratio of magnesium ion: A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: This is two positive charges and two negative charges. Now consider the ionic compound formed by magnesium and chlorine. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. 2 and. Chloride Ion In Magnesium.
From www.slideserve.com
PPT Visualizing a Chemical Reaction PowerPoint Presentation, free Chloride Ion In Magnesium Thus, the ratio of magnesium ion: 2 and the simplest magnesium. This is two positive charges and two negative charges. So, magnesium ions form the ionic bond with two chlorine ions. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The slideshow shows dot and cross diagrams for the ions in sodium chloride,. Chloride Ion In Magnesium.
From favpng.com
Lewis Structure Magnesium Chloride Electron Diagram, PNG, 1024x1024px Chloride Ion In Magnesium 2 and the simplest magnesium. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Thus, the ratio of magnesium ion: At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties.. Chloride Ion In Magnesium.
From www.slideserve.com
PPT Salts and Solubility PowerPoint Presentation, free download ID Chloride Ion In Magnesium It is an ionic compound, composed. This is two positive charges and two negative charges. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. So, magnesium ions form the ionic bond with two chlorine ions. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. A. Chloride Ion In Magnesium.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Chloride Ion In Magnesium Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. Thus, the ratio of magnesium ion: About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. This is. Chloride Ion In Magnesium.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chloride Ion In Magnesium About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. This is two positive charges and two negative charges. It is an ionic compound, composed. At the heart of magnesium chloride’s usefulness is. Chloride Ion In Magnesium.
From beautyambassade.com
Magnesium Chloride Skincare ingredient Skin care products Chloride Ion In Magnesium So, magnesium ions form the ionic bond with two chlorine ions. It is an ionic compound, composed. Now consider the ionic compound formed by magnesium and chlorine. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. A magnesium ion has a 2+ charge, while a chlorine ion has a 1−. Chloride Ion In Magnesium.
From slidetodoc.com
SSLC ONLINE CLASSES Grade 10 Chapter 3 Subject Chloride Ion In Magnesium This is two positive charges and two negative charges. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Now consider the ionic compound formed by magnesium and chlorine. It is an ionic compound, composed. 2 and the simplest magnesium. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺). Chloride Ion In Magnesium.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Chloride Ion In Magnesium This is two positive charges and two negative charges. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Thus, the ratio of magnesium ion: So, magnesium ions form the ionic bond with two chlorine ions. Now consider the ionic compound formed by magnesium and chlorine. 2 and the simplest magnesium. A magnesium ion has. Chloride Ion In Magnesium.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Chloride Ion In Magnesium 2 and the simplest magnesium. It is an ionic compound, composed. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. So, magnesium ions form the ionic bond with two chlorine ions. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. This is two positive charges and two. Chloride Ion In Magnesium.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Chloride Ion In Magnesium The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. So, magnesium ions form the ionic bond with two chlorine ions. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: It is an ionic compound, composed. At the heart of magnesium chloride’s usefulness is its unique. Chloride Ion In Magnesium.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chloride Ion In Magnesium 2 and the simplest magnesium. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Now consider the ionic compound formed by magnesium and chlorine. At the heart of magnesium chloride’s usefulness is its unique set. Chloride Ion In Magnesium.
From socratic.org
Question 15936 + Example Chloride Ion In Magnesium A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 2 and the simplest magnesium. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Thus, the ratio of magnesium ion: The slideshow shows. Chloride Ion In Magnesium.
From ar.inspiredpencil.com
Magnesium Chloride Lewis Dot Structure Chloride Ion In Magnesium Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. This is two positive charges and two negative charges. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a. Chloride Ion In Magnesium.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Chloride Ion In Magnesium So, magnesium ions form the ionic bond with two chlorine ions. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: It is an ionic compound, composed. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride,. Chloride Ion In Magnesium.
From www.youtube.com
Is MgCl2 (Magnesium chloride) Ionic or Covalent? YouTube Chloride Ion In Magnesium A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. 2 and the simplest magnesium. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. So, magnesium ions form the ionic. Chloride Ion In Magnesium.
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Chloride Ion In Magnesium A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Thus, the ratio of magnesium ion: Now consider the ionic. Chloride Ion In Magnesium.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Chloride Ion In Magnesium About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. 2 and the simplest magnesium. Now consider the ionic compound formed by magnesium and chlorine. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. A magnesium ion has a 2+ charge, while a chlorine ion. Chloride Ion In Magnesium.
From ar.inspiredpencil.com
Magnesium Chloride Structure Chloride Ion In Magnesium At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. 2 and the simplest magnesium. Now consider the ionic compound formed by magnesium and chlorine. A magnesium ion has a 2+ charge, while a chlorine. Chloride Ion In Magnesium.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Chloride Ion In Magnesium 2 and the simplest magnesium. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. Thus, the ratio of magnesium ion: So,. Chloride Ion In Magnesium.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Chloride Ion In Magnesium So, magnesium ions form the ionic bond with two chlorine ions. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions.. Chloride Ion In Magnesium.
From www.toppr.com
How is magnesium chloride formed by the transfer of electrons? Why does Chloride Ion In Magnesium Now consider the ionic compound formed by magnesium and chlorine. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. It is an ionic compound, composed. Thus, the ratio of magnesium ion: At the heart of magnesium. Chloride Ion In Magnesium.
From www.slideshare.net
2012 topic 4.1 bonding ionic Chloride Ion In Magnesium 2 and the simplest magnesium. It is an ionic compound, composed. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. So, magnesium ions form the ionic bond with two chlorine ions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. A magnesium ion has. Chloride Ion In Magnesium.
From www.youtube.com
Ionic bonding Sodium chloride,Magnesium chloride Class 10 Chloride Ion In Magnesium Now consider the ionic compound formed by magnesium and chlorine. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: 2 and the simplest magnesium. This is two positive charges and two negative charges. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. At the heart of magnesium chloride’s. Chloride Ion In Magnesium.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Chloride Ion In Magnesium About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Thus, the ratio of magnesium ion: The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. Now consider the ionic compound formed by magnesium and chlorine. 2 and the simplest magnesium. A magnesium. Chloride Ion In Magnesium.
From www.benjamin-mills.com
Electron configurations Chloride Ion In Magnesium A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: So, magnesium ions form the ionic bond with two chlorine ions. This is two positive charges and two negative charges. 2 and the simplest magnesium. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. It is. Chloride Ion In Magnesium.
From techiescientist.com
Is MgCl2 Ionic or Covalent? Techiescientist Chloride Ion In Magnesium At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Now consider the ionic compound formed by magnesium and chlorine. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. 2 and the simplest magnesium. So, magnesium ions form the ionic bond with two chlorine ions.. Chloride Ion In Magnesium.