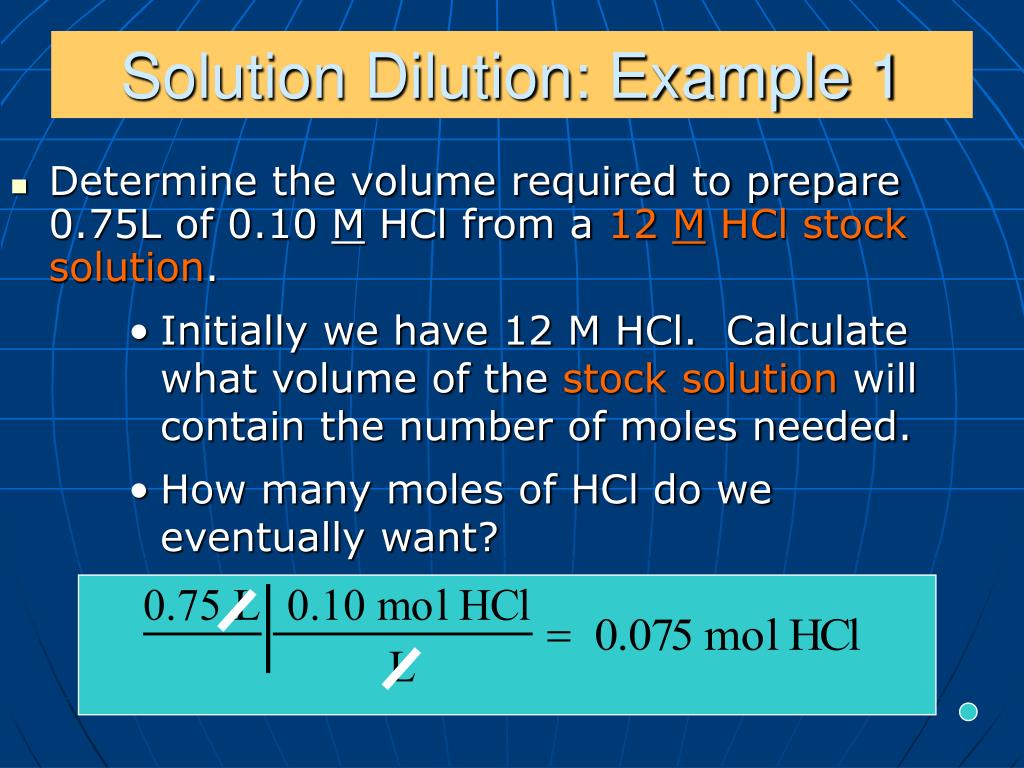
Dilution Of Solution Example . Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Make 300 μl of a 1:250 dilution. This can be done by adding solvent to the main bulk of the solution or taking some. Dilution is the addition of. Learn how to dilute and concentrate solutions. Final volume / solute volume = df. (300 μl) / solute volume =. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume.
from www.slideserve.com
Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. (300 μl) / solute volume =. Final volume / solute volume = df. This can be done by adding solvent to the main bulk of the solution or taking some. Dilution is the addition of. Make 300 μl of a 1:250 dilution. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute.
PPT Chapter 13 PowerPoint Presentation, free download ID5812279
Dilution Of Solution Example (300 μl) / solute volume =. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. (300 μl) / solute volume =. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Final volume / solute volume = df. Dilution is the addition of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This can be done by adding solvent to the main bulk of the solution or taking some. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Make 300 μl of a 1:250 dilution.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free Dilution Of Solution Example State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. Final volume / solute volume = df. Make 300 μl of a 1:250 dilution. (300 μl) / solute volume =. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more. Dilution Of Solution Example.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Of Solution Example Learn how to dilute and concentrate solutions. This can be done by adding solvent to the main bulk of the solution or taking some. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is. Dilution Of Solution Example.
From spmchemistry.blog.onlinetuition.com.my
Dilution SPM Chemistry Dilution Of Solution Example Dilution is the addition of. Learn how to dilute and concentrate solutions. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. (300 μl) / solute volume =. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.. Dilution Of Solution Example.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilution Of Solution Example Final volume / solute volume = df. Dilution is the addition of. (300 μl) / solute volume =. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. This. Dilution Of Solution Example.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution Of Solution Example Final volume / solute volume = df. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the. Dilution Of Solution Example.
From www.slideserve.com
PPT Solutions & Dilutions PowerPoint Presentation, free download ID Dilution Of Solution Example Often, a worker will need to change the concentration of a solution by changing the amount of solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. (300 μl) / solute volume =. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Dilution Of Solution Example.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Of Solution Example Dilution is the addition of. Make 300 μl of a 1:250 dilution. (300 μl) / solute volume =. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker. Dilution Of Solution Example.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilution Of Solution Example Learn how to dilute and concentrate solutions. (300 μl) / solute volume =. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Make 300 μl of a 1:250 dilution. A dilution is a process where the concentration. Dilution Of Solution Example.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilution Of Solution Example A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This can be done by adding solvent to the main bulk of the solution or taking some. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. State whether. Dilution Of Solution Example.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Of Solution Example Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Learn how to dilute and concentrate solutions. (300 μl) / solute volume =. This can be done by adding solvent to the main bulk of the solution or taking some. A dilution is a process where the concentration of a solution is lowered. Dilution Of Solution Example.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Of Solution Example Make 300 μl of a 1:250 dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. (300 μl) / solute volume =. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. This can be done by adding solvent to the. Dilution Of Solution Example.
From www.slideserve.com
PPT Chapter 13. Solutions PowerPoint Presentation, free download ID Dilution Of Solution Example Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. (300 μl) / solute volume =. This can be done by adding solvent to the main bulk of the solution or taking some. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution. Dilution Of Solution Example.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Of Solution Example Dilution is the addition of. Learn how to dilute and concentrate solutions. Final volume / solute volume = df. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as.. Dilution Of Solution Example.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation, free Dilution Of Solution Example Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Final volume / solute volume = df. Dilution is the process of. Dilution Of Solution Example.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Of Solution Example State whether the concentration of a solution is directly or indirectly proportional to its volume. Final volume / solute volume = df. Make 300 μl of a 1:250 dilution. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Learn how to dilute and concentrate solutions. This can be done by adding solvent. Dilution Of Solution Example.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Of Solution Example (300 μl) / solute volume =. Learn how to dilute and concentrate solutions. Dilution is the addition of. This can be done by adding solvent to the main bulk of the solution or taking some. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Make 300. Dilution Of Solution Example.
From labpedia.net
Solutions Part 2 Preparation of solutions (Molar, Normal) and Dilution Of Solution Example (300 μl) / solute volume =. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Learn how to dilute and concentrate solutions. Final volume / solute volume = df. Often, a worker will need to change the concentration of a solution by changing the amount of. Dilution Of Solution Example.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Of Solution Example Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. This can be done by adding solvent to the main bulk of the solution or taking some. Dilution is the addition of. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Dilution Of Solution Example.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID Dilution Of Solution Example Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. This can be done by adding solvent to the main bulk of the solution or taking some. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Dilution Of Solution Example.
From www.youtube.com
U10L4 Molarity, Dilution, PPM, and Molality Calculations YouTube Dilution Of Solution Example Often, a worker will need to change the concentration of a solution by changing the amount of solvent. (300 μl) / solute volume =. Learn how to dilute and concentrate solutions. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. This can be done by. Dilution Of Solution Example.
From www.slideserve.com
PPT Chapter 16 Solutions PowerPoint Presentation, free download ID Dilution Of Solution Example Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Often, a worker will need to change the concentration. Dilution Of Solution Example.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilution Of Solution Example Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Make 300 μl of a 1:250 dilution. Final volume / solute volume = df. Learn how to dilute and concentrate solutions. A dilution is a. Dilution Of Solution Example.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution Of Solution Example Often, a worker will need to change the concentration of a solution by changing the amount of solvent. (300 μl) / solute volume =. Learn how to dilute and concentrate solutions. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Make 300 μl of a. Dilution Of Solution Example.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilution Of Solution Example Dilution is the addition of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. This can be done by adding solvent to the main bulk of the solution or taking some.. Dilution Of Solution Example.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilution Of Solution Example A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the addition of. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Diluting a solvent means to lower the concentration. Dilution Of Solution Example.
From labpedia.net
Solutions Part 3 Solutions with various examples Dilution Of Solution Example Make 300 μl of a 1:250 dilution. Learn how to dilute and concentrate solutions. Final volume / solute volume = df. State whether the concentration of a solution is directly or indirectly proportional to its volume. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. A dilution is a process where the. Dilution Of Solution Example.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Of Solution Example Learn how to dilute and concentrate solutions. Make 300 μl of a 1:250 dilution. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Final volume / solute volume. Dilution Of Solution Example.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Of Solution Example Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Final volume / solute volume = df. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. This can be done. Dilution Of Solution Example.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Of Solution Example A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Often, a worker will need to change the concentration of a solution by. Dilution Of Solution Example.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilution Of Solution Example Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Often, a worker will need to change the concentration of a solution by. Dilution Of Solution Example.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Of Solution Example Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Often, a worker will need to change the concentration of a solution by. Dilution Of Solution Example.
From www.thoughtco.com
Dilution Calculations From Stock Solutions in Chemistry Dilution Of Solution Example Learn how to dilute and concentrate solutions. Final volume / solute volume = df. Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Make 300 μl of a 1:250. Dilution Of Solution Example.
From www.youtube.com
TYPES OF SOLUTION,Dilution of Solution,Concentrated,Dilute solutions Dilution Of Solution Example Dilution is the addition of. Final volume / solute volume = df. This can be done by adding solvent to the main bulk of the solution or taking some. Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Diluting. Dilution Of Solution Example.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilution Of Solution Example Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a process where the concentration of a solution is lowered by adding solvent to the. Dilution Of Solution Example.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Of Solution Example Diluting a solvent means to lower the concentration by adding more of the solvent to the solution. Make 300 μl of a 1:250 dilution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. This can be done by adding solvent to the main bulk of. Dilution Of Solution Example.