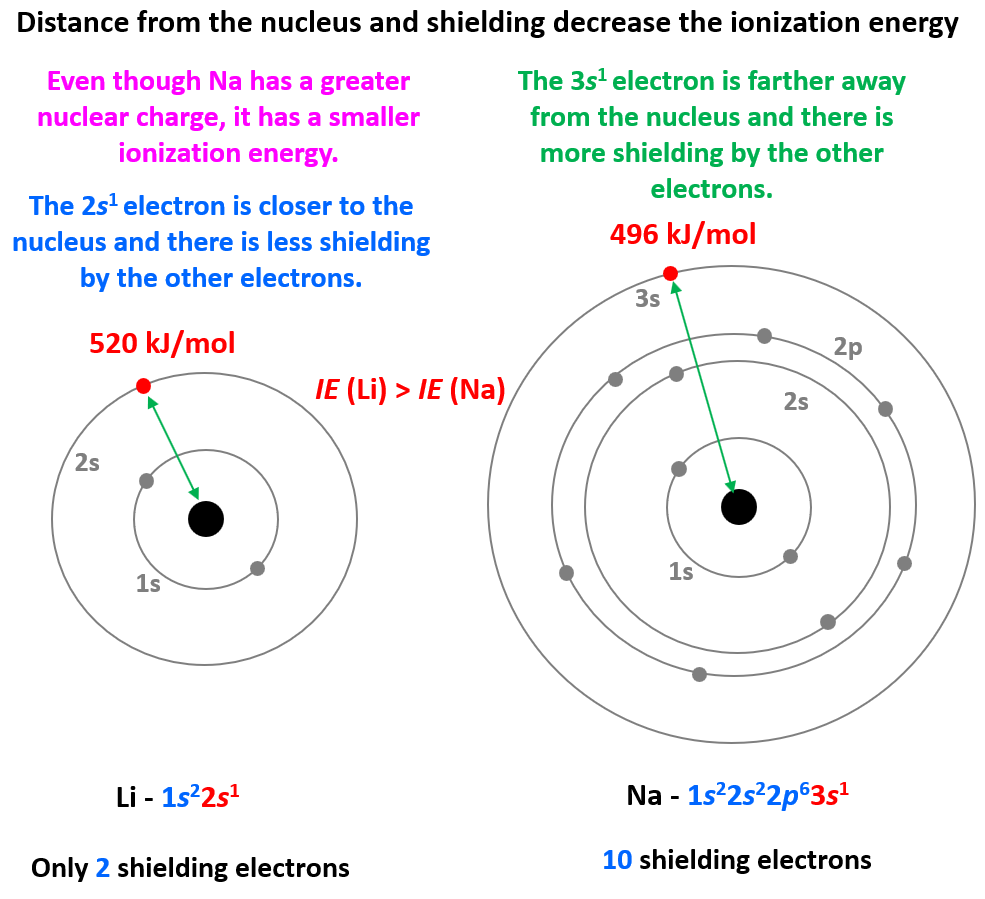
Magnesium Shielding Electrons . What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? So the number of shielding electrons for. The first ionisation energy of.
from general.chemistrysteps.com
Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. The first ionisation energy of. So the number of shielding electrons for. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?
Ionization energy Chemistry Steps
Magnesium Shielding Electrons So the number of shielding electrons for. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. The first ionisation energy of. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. So the number of shielding electrons for. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?
From www.dreamstime.com
Magnesium Atom, with Mass and Energy Levels. Stock Vector Illustration of vector, shell 254497393 Magnesium Shielding Electrons Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? Learn how to calculate the effect of shielding on electrons. Magnesium Shielding Electrons.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Magnesium Shielding Electrons The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. The first ionisation energy of. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. The first ionisation energy is the energy involved in. Magnesium Shielding Electrons.
From www.dreamstime.com
Atom Of Magnesium With Detailed Core And Its 12 Electrons Stock Illustration Illustration of Magnesium Shielding Electrons Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. What is the effective attraction \(z_{eff}\) experienced by the. Magnesium Shielding Electrons.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Shielding Electrons Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. The first. Magnesium Shielding Electrons.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. So the number of shielding electrons for. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. Learn how to calculate the effect of shielding. Magnesium Shielding Electrons.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium YouTube Magnesium Shielding Electrons So the number of shielding electrons for. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. The first ionisation energy of. The first ionisation energy is the energy involved. Magnesium Shielding Electrons.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Magnesium Shielding Electrons So the number of shielding electrons for. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms. Magnesium Shielding Electrons.
From www.dreamstime.com
Electron of the Element Magnesium Stock Vector Illustration of representation, atom 251040511 Magnesium Shielding Electrons The first ionisation energy of. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. So the number of shielding electrons for. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. See examples of. Magnesium Shielding Electrons.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download ID3646738 Magnesium Shielding Electrons The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. So the number of shielding electrons for. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. The first ionisation energy of. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. Magnesium Shielding Electrons.
From socratic.org
How are shielding effect and atomic radius related? Socratic Magnesium Shielding Electrons The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. The first ionisation energy of. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real. Magnesium Shielding Electrons.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Magnesium Shielding Electrons The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium. Magnesium Shielding Electrons.
From www.dreamstime.com
Diagram Representation Of The Element Magnesium Stock Vector Image 59013293 Magnesium Shielding Electrons The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge. Magnesium Shielding Electrons.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Shielding Electrons The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. So the number of shielding electrons for. The electron. Magnesium Shielding Electrons.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron configuration, chemical data, and Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. So the number of shielding electrons for. What is the effective attraction \(z_{eff}\) experienced by. Magnesium Shielding Electrons.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Shielding Electrons See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. The first ionisation energy of. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the. Magnesium Shielding Electrons.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Magnesium Shielding Electrons The first ionisation energy of. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. So the number of shielding electrons for. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? See examples of shielding effects in lithium, magnesium,. Magnesium Shielding Electrons.
From www.numerade.com
SOLVED In which orbital does an electron in a magnesium atom experience the greatest shielding Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the. Magnesium Shielding Electrons.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Periodic Table of the Elements Magnesium Shielding Electrons The first ionisation energy of. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. So the number of. Magnesium Shielding Electrons.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion,. Magnesium Shielding Electrons.
From www.dreamstime.com
Atom of Magnesium with Detailed Core and 12 Electrons on White with Atoms Stock Illustration Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning. Magnesium Shielding Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. The first ionisation energy. Magnesium Shielding Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? So the number of shielding electrons for. The first ionisation energy of. See examples of shielding. Magnesium Shielding Electrons.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Protons are represented Magnesium Shielding Electrons The first ionisation energy of. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? So the number of shielding electrons for. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. See examples of shielding effects in lithium, magnesium,. Magnesium Shielding Electrons.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Magnesium Shielding Electrons The first ionisation energy of. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium. Magnesium Shielding Electrons.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? So the number of shielding electrons for. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. Magnesium Shielding Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Shielding Electrons The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion,. Magnesium Shielding Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Shielding Electrons What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. See examples of shielding effects in lithium, magnesium, aluminum, sodium. Magnesium Shielding Electrons.
From commons.wikimedia.org
FileElectron shell 012 Magnesium.svg Wikimedia Commons Magnesium Shielding Electrons Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. The first ionisation energy is the energy involved in removing one mole of electrons. Magnesium Shielding Electrons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Shielding Electrons So the number of shielding electrons for. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium. Magnesium Shielding Electrons.
From www.youtube.com
Valence Electrons for Magnesium (Mg) YouTube Magnesium Shielding Electrons Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. What is the effective attraction \(z_{eff}\) experienced by the. Magnesium Shielding Electrons.
From www.youtube.com
What does higher shielding effect mean Which element has the highest shielding effect? YouTube Magnesium Shielding Electrons The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the. Magnesium Shielding Electrons.
From brainly.com
a single 3s electron in a magnesium atom is shielded by a) the 2s and the 2p electrons only b Magnesium Shielding Electrons Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. So the number of shielding electrons for. What is the effective attraction \(z_{eff}\) experienced. Magnesium Shielding Electrons.
From guidelibstoitering.z21.web.core.windows.net
Magnesium Electron Dot Diagram Magnesium Shielding Electrons The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation? What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion,. Magnesium Shielding Electrons.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Shielding Electrons So the number of shielding electrons for. The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state. Learn how to calculate the effect of shielding on electrons with our. Magnesium Shielding Electrons.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Shielding Electrons The electron configuration for magnesium is {eq}[ne] 3s^2 {/eq}, meaning an atom of magnesium has 2 valence electrons. See examples of shielding effects in lithium, magnesium, aluminum, sodium and cesium. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium atom, and magnesium cation?. The first ionisation energy is the energy involved. Magnesium Shielding Electrons.