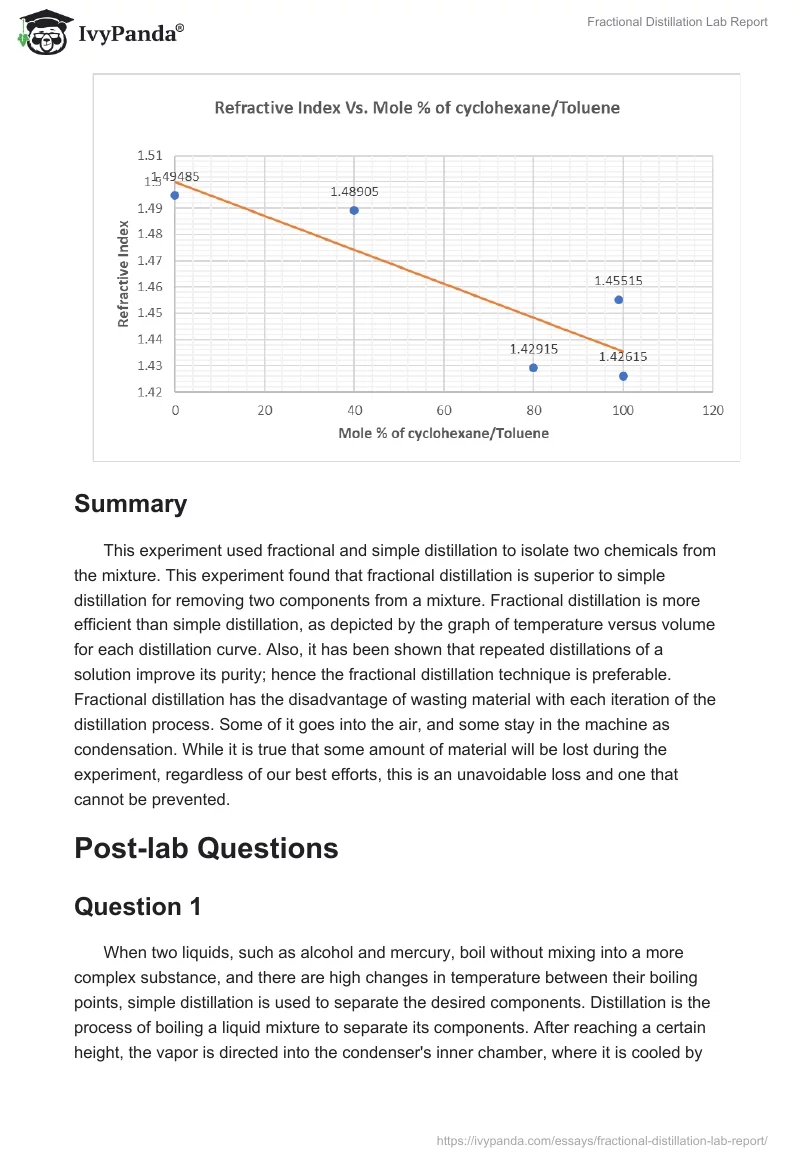
Simple And Fractional Distillation Lab Report Discussion . Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. The most common methods of distillation are simple distillation and fractional distillation. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. In this experiment, we will separate 2 distillates using their differences in boiling points. The boiling points of individual. Simple simple distillation can be used when the. The vapor rises and passes into a condenser. Separating two miscible liquids through simple and fractional distillation. The overall goal of this experiment was to separate two miscible.
from ivypanda.com
Simple simple distillation can be used when the. The vapor rises and passes into a condenser. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Separating two miscible liquids through simple and fractional distillation. In this experiment, we will separate 2 distillates using their differences in boiling points. The most common methods of distillation are simple distillation and fractional distillation. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The overall goal of this experiment was to separate two miscible. The boiling points of individual. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic.
Fractional Distillation Lab Report 756 Words Report Example
Simple And Fractional Distillation Lab Report Discussion The overall goal of this experiment was to separate two miscible. The overall goal of this experiment was to separate two miscible. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. The most common methods of distillation are simple distillation and fractional distillation. Separating two miscible liquids through simple and fractional distillation. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The boiling points of individual. In this experiment, we will separate 2 distillates using their differences in boiling points. Simple simple distillation can be used when the. The vapor rises and passes into a condenser.
From exocnnftw.blob.core.windows.net
Simple Distillation Lab Report Discussion at Blanche Merritt blog Simple And Fractional Distillation Lab Report Discussion The boiling points of individual. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The most common methods of distillation are simple distillation and fractional distillation. The vapor rises and passes into a condenser. The overall goal of this experiment was to separate two miscible. Distillation is one of the oldest and. Simple And Fractional Distillation Lab Report Discussion.
From exoevcgtp.blob.core.windows.net
Fractional Distillation Lab Report Discussion at Bill Fyffe blog Simple And Fractional Distillation Lab Report Discussion In this experiment, we will separate 2 distillates using their differences in boiling points. The overall goal of this experiment was to separate two miscible. Separating two miscible liquids through simple and fractional distillation. Simple simple distillation can be used when the. The boiling points of individual. Fractional distillation is used because it can complete several little distillations, or theoretical. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Haile. simple distillation lab report MTSU Lab report Template for Simple And Fractional Distillation Lab Report Discussion The most common methods of distillation are simple distillation and fractional distillation. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The boiling points of individual. In this experiment, we will separate 2 distillates using their differences in boiling points. The vapor rises and passes into a condenser. Simple simple distillation can. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
CH121 Exp2 Simple and Fractional Distillation EXPERIMENT 2 SIMPLE Simple And Fractional Distillation Lab Report Discussion The most common methods of distillation are simple distillation and fractional distillation. In this experiment, we will separate 2 distillates using their differences in boiling points. The boiling points of individual. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. Separating two miscible liquids through simple and fractional distillation.. Simple And Fractional Distillation Lab Report Discussion.
From psiberg.com
Simple vs. Fractional Distillation A Comparison Simple And Fractional Distillation Lab Report Discussion Simple simple distillation can be used when the. In this experiment, we will separate 2 distillates using their differences in boiling points. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. The boiling points of individual. In simple distillation, a mixture is boiled to change the most volatile component. Simple And Fractional Distillation Lab Report Discussion.
From paperap.com
Distillation Experiment Conclusion Report Conclusion Essay Example Simple And Fractional Distillation Lab Report Discussion Separating two miscible liquids through simple and fractional distillation. In this experiment, we will separate 2 distillates using their differences in boiling points. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The overall goal of this experiment was to separate two miscible. Distillation is one of the oldest and still most. Simple And Fractional Distillation Lab Report Discussion.
From www.chegg.com
Solved Conclusion In the conclusion the aim of experiment Simple And Fractional Distillation Lab Report Discussion In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Simple simple distillation can be used when the. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The vapor rises and passes into a condenser. Distillation is one of the oldest and still most. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Lab 1 Report Simple vs. Fractional Distillation Results and Simple And Fractional Distillation Lab Report Discussion Separating two miscible liquids through simple and fractional distillation. The most common methods of distillation are simple distillation and fractional distillation. The overall goal of this experiment was to separate two miscible. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. Simple simple distillation can be used when the. The vapor rises. Simple And Fractional Distillation Lab Report Discussion.
From www.academia.edu
(DOC) Sample Lab Report Simple and Fractional Distillation Nur Simple And Fractional Distillation Lab Report Discussion The vapor rises and passes into a condenser. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. In this experiment, we will separate 2 distillates using their differences in boiling points. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor.. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Lab 1 Simple & Fractional Distillations of a Mixture Water & Methanol Simple And Fractional Distillation Lab Report Discussion In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. The most common methods of distillation are simple distillation and fractional distillation. In this experiment, we will separate 2 distillates using their differences in boiling points. Distillation is one of the oldest and still most common methods for both the purification and. Simple And Fractional Distillation Lab Report Discussion.
From www.teachoo.com
Difference between Distillation and Fractional Distillation in Table Simple And Fractional Distillation Lab Report Discussion The boiling points of individual. The vapor rises and passes into a condenser. The overall goal of this experiment was to separate two miscible. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Simple simple distillation can be used when the. Fractional distillation is used because it can complete several little. Simple And Fractional Distillation Lab Report Discussion.
From www.edrawmax.com
Distillation Lab Report EdrawMax Template Simple And Fractional Distillation Lab Report Discussion Simple simple distillation can be used when the. The most common methods of distillation are simple distillation and fractional distillation. In this experiment, we will separate 2 distillates using their differences in boiling points. The vapor rises and passes into a condenser. Distillation is one of the oldest and still most common methods for both the purification and the identification. Simple And Fractional Distillation Lab Report Discussion.
From ivypanda.com
Fractional Distillation Lab Report 756 Words Report Example Simple And Fractional Distillation Lab Report Discussion Separating two miscible liquids through simple and fractional distillation. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The vapor rises and passes into a condenser. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. Simple simple distillation can be used. Simple And Fractional Distillation Lab Report Discussion.
From www.chegg.com
Solved EXPERIMENT 6 Simple and Fractional Distillation Simple And Fractional Distillation Lab Report Discussion The overall goal of this experiment was to separate two miscible. The most common methods of distillation are simple distillation and fractional distillation. In this experiment, we will separate 2 distillates using their differences in boiling points. The vapor rises and passes into a condenser. Distillation is one of the oldest and still most common methods for both the purification. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Lab 1 Report TA Experiment 1 Simple and Fractional Distillation Simple And Fractional Distillation Lab Report Discussion The overall goal of this experiment was to separate two miscible. Separating two miscible liquids through simple and fractional distillation. The most common methods of distillation are simple distillation and fractional distillation. The boiling points of individual. Simple simple distillation can be used when the. In simple distillation, a mixture is boiled to change the most volatile component from a. Simple And Fractional Distillation Lab Report Discussion.
From www.docsity.com
Fractional distillation lab report Study Guides, Projects, Research Simple And Fractional Distillation Lab Report Discussion The vapor rises and passes into a condenser. The overall goal of this experiment was to separate two miscible. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The boiling points of individual. The most common methods of distillation are simple distillation and fractional distillation. In simple distillation, a mixture is boiled. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Experiment 2 Simple and Fractional Distillation Determination of Simple And Fractional Distillation Lab Report Discussion In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Separating two miscible liquids through simple and fractional distillation. The overall goal of this experiment was to separate two miscible. The boiling points of individual. The vapor rises and passes into a condenser. Fractional distillation is used because it can complete several. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Distillation Lab Report 0 Simple and Fractional Distillation Aim To Simple And Fractional Distillation Lab Report Discussion The boiling points of individual. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Separating two miscible liquids through simple and fractional distillation. The overall goal of this experiment was to separate two miscible. The most common methods of distillation are simple distillation and fractional distillation. Distillation is one of the. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Distillation Lab lab Simple and Fractional Distillation Simple And Fractional Distillation Lab Report Discussion In this experiment, we will separate 2 distillates using their differences in boiling points. The overall goal of this experiment was to separate two miscible. The boiling points of individual. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. Separating two miscible liquids through simple and fractional distillation. Simple. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Lab Report 3Simple, Fractional, Steam Distillation Lab Lab Simple Simple And Fractional Distillation Lab Report Discussion Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. The boiling points of individual. In this experiment, we will separate 2 distillates using their differences in boiling points. Separating two miscible liquids through simple. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Orgo1 Lab Report Simple and Fractional distillation Stephany Frantser Simple And Fractional Distillation Lab Report Discussion Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. Separating two miscible liquids through simple and fractional distillation. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. Simple simple distillation can be used when the. The overall goal of this experiment. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Separation of Liquids Fractional Distillation and Gas Chromatography Simple And Fractional Distillation Lab Report Discussion The overall goal of this experiment was to separate two miscible. The boiling points of individual. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Separating two miscible liquids through simple and fractional distillation.. Simple And Fractional Distillation Lab Report Discussion.
From www.docsity.com
Simple and fractional distillation lab report Study Guides, Projects Simple And Fractional Distillation Lab Report Discussion Simple simple distillation can be used when the. The most common methods of distillation are simple distillation and fractional distillation. Separating two miscible liquids through simple and fractional distillation. The overall goal of this experiment was to separate two miscible. The boiling points of individual. In this experiment, we will separate 2 distillates using their differences in boiling points. The. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Post Lab Report 2 Distillation Lab Report 2 Simple and Fractional Simple And Fractional Distillation Lab Report Discussion In this experiment, we will separate 2 distillates using their differences in boiling points. The most common methods of distillation are simple distillation and fractional distillation. The boiling points of individual. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. Simple simple distillation can be used when the. The overall goal of. Simple And Fractional Distillation Lab Report Discussion.
From exocnnftw.blob.core.windows.net
Simple Distillation Lab Report Discussion at Blanche Merritt blog Simple And Fractional Distillation Lab Report Discussion Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. The boiling points of individual. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor.. Simple And Fractional Distillation Lab Report Discussion.
From www.chegg.com
Solved EXPERIMENT 6 Simple and Fractional Distillation Simple And Fractional Distillation Lab Report Discussion Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. The boiling points of individual. The overall goal of this experiment was to separate two miscible. The vapor rises and passes into a condenser. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Orgo I lab report 1 1.25, CHEM 3105 TA Simple and Fractional Simple And Fractional Distillation Lab Report Discussion Separating two miscible liquids through simple and fractional distillation. In this experiment, we will separate 2 distillates using their differences in boiling points. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. The overall goal of this experiment was to separate two miscible. The boiling points of individual. The. Simple And Fractional Distillation Lab Report Discussion.
From exoevcgtp.blob.core.windows.net
Fractional Distillation Lab Report Discussion at Bill Fyffe blog Simple And Fractional Distillation Lab Report Discussion Separating two miscible liquids through simple and fractional distillation. The overall goal of this experiment was to separate two miscible. In this experiment, we will separate 2 distillates using their differences in boiling points. Simple simple distillation can be used when the. Distillation is one of the oldest and still most common methods for both the purification and the identification. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Lab report simple and fractional distillation Experiment Simple and Simple And Fractional Distillation Lab Report Discussion The most common methods of distillation are simple distillation and fractional distillation. Separating two miscible liquids through simple and fractional distillation. The boiling points of individual. In this experiment, we will separate 2 distillates using their differences in boiling points. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Fractional distillation. Simple And Fractional Distillation Lab Report Discussion.
From ivypanda.com
Fractional Distillation Lab Report 756 Words Report Example Simple And Fractional Distillation Lab Report Discussion In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. The overall goal of this experiment was to separate two miscible. The vapor rises and passes into a condenser. In this experiment, we will separate 2 distillates using their differences in boiling points. The boiling points of individual. The most common methods. Simple And Fractional Distillation Lab Report Discussion.
From studylib.net
Simple and Fractional Distillation Lab Report Simple And Fractional Distillation Lab Report Discussion Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. Simple simple distillation can be used when the. In simple distillation, a mixture is boiled to change the most volatile component from a liquid into vapor. Separating two miscible liquids through simple and fractional distillation. Distillation is one of the oldest and still. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Lab report week 3 pdf Simple and Fractional Distillation for a Simple And Fractional Distillation Lab Report Discussion In this experiment, we will separate 2 distillates using their differences in boiling points. The vapor rises and passes into a condenser. Simple simple distillation can be used when the. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. The boiling points of individual. Separating two miscible liquids through. Simple And Fractional Distillation Lab Report Discussion.
From www.studocu.com
Simple and Fractional Distillation Lab Report Rosita Beatriz Ramirez Simple And Fractional Distillation Lab Report Discussion Separating two miscible liquids through simple and fractional distillation. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. Simple simple distillation can be used when the. The boiling points of individual. The overall goal of this experiment was to separate two miscible. In simple distillation, a mixture is boiled. Simple And Fractional Distillation Lab Report Discussion.
From ivypanda.com
Fractional Distillation Lab Report 756 Words Report Example Simple And Fractional Distillation Lab Report Discussion In this experiment, we will separate 2 distillates using their differences in boiling points. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The boiling points of individual. The vapor rises and passes into a condenser. The overall goal of this experiment was to separate two miscible. Simple simple distillation can be. Simple And Fractional Distillation Lab Report Discussion.
From exoevcgtp.blob.core.windows.net
Fractional Distillation Lab Report Discussion at Bill Fyffe blog Simple And Fractional Distillation Lab Report Discussion Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. The boiling points of individual. In this experiment, we will separate 2 distillates using their differences in boiling points. In simple distillation, a. Simple And Fractional Distillation Lab Report Discussion.