When Water Starts Boiling The Temperature . boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; These changes are discussed further in a section below. Though it’s one of the basic facts you probably learnt pretty early on. water always boils at 100˚c, right? the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. At other elevations the melting point will change due to different ambient pressure. boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas.
from www.chegg.com
At other elevations the melting point will change due to different ambient pressure. boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Though it’s one of the basic facts you probably learnt pretty early on. These changes are discussed further in a section below. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). water always boils at 100˚c, right? the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid;
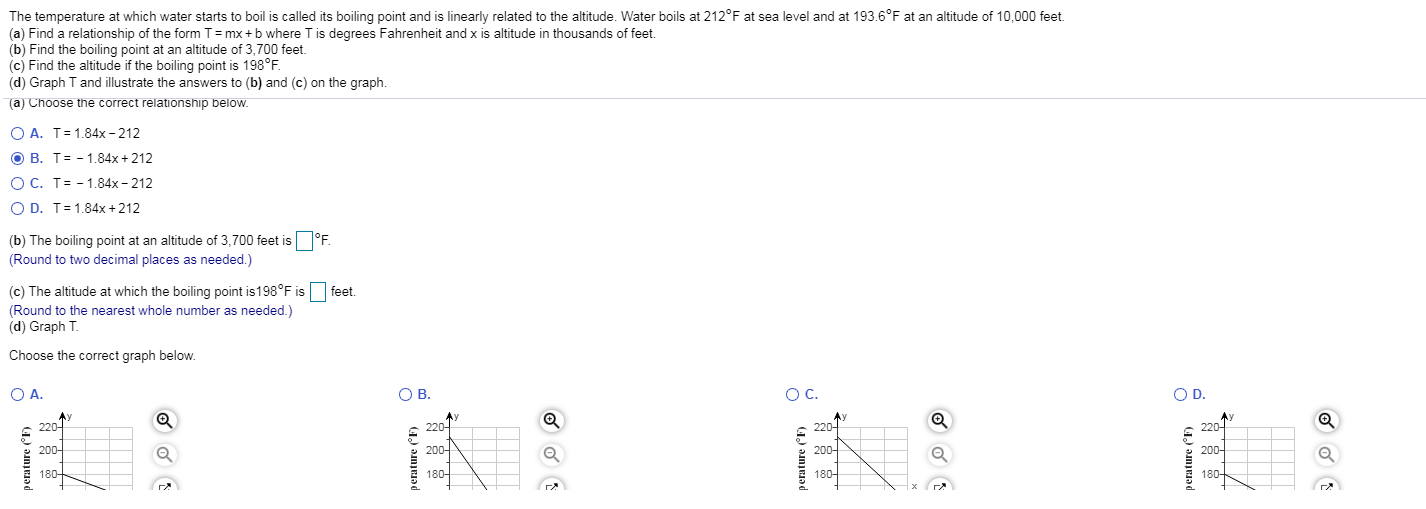
Solved The temperature at which water starts to boil is
When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; At other elevations the melting point will change due to different ambient pressure. Though it’s one of the basic facts you probably learnt pretty early on. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). These changes are discussed further in a section below. boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; water always boils at 100˚c, right?
From sciencenotes.org
How to Boil Water at Room Temperature When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; At other elevations the melting point will change due to different ambient pressure. Though it’s one of the basic facts you probably learnt pretty early on. water always boils at 100˚c, right?. When Water Starts Boiling The Temperature.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). These changes are discussed further in a section below. At other. When Water Starts Boiling The Temperature.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Vector Adobe Stock When Water Starts Boiling The Temperature Though it’s one of the basic facts you probably learnt pretty early on. These changes are discussed further in a section below. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. water always boils at 100˚c, right? boiling occurs when bubbles. When Water Starts Boiling The Temperature.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation When Water Starts Boiling The Temperature water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. water always boils at 100˚c, right? These changes are discussed further in a section below. boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid. When Water Starts Boiling The Temperature.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit? HowChimp When Water Starts Boiling The Temperature water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; At other elevations the melting point will. When Water Starts Boiling The Temperature.
From sciencenotes.org
How to Boil Water at Room Temperature When Water Starts Boiling The Temperature water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Though it’s one of the basic facts you probably learnt pretty early on. These changes are discussed further in a section below. boiling point, temperature at which the pressure exerted by the surroundings. When Water Starts Boiling The Temperature.
From www.youtube.com
Boiling temperature of water? Seen from a freeze drying perspective YouTube When Water Starts Boiling The Temperature water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. water always boils at 100˚c, right? boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. boiling point, temperature at. When Water Starts Boiling The Temperature.
From hikingmastery.com
Does Boiling Water Purify It Basic Facts and Useful When Water Starts Boiling The Temperature the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. water always boils at 100˚c, right? At other elevations the melting point will change due to different ambient pressure. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for. When Water Starts Boiling The Temperature.
From exojcdfpu.blob.core.windows.net
How Do You Get Water To Boil Faster at Judy blog When Water Starts Boiling The Temperature the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. water always boils at 100˚c, right? boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. boiling point, temperature at which. When Water Starts Boiling The Temperature.
From www.worksheetsplanet.com
What is Boiling Point When Water Starts Boiling The Temperature water always boils at 100˚c, right? the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). Though it’s one of the basic. When Water Starts Boiling The Temperature.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER When Water Starts Boiling The Temperature At other elevations the melting point will change due to different ambient pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level. When Water Starts Boiling The Temperature.
From www.chegg.com
Solved The temperature at which water starts to boil is When Water Starts Boiling The Temperature These changes are discussed further in a section below. boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. water always boils. When Water Starts Boiling The Temperature.
From dxopohezx.blob.core.windows.net
What Is Water S Boiling Temp at Greg Clark blog When Water Starts Boiling The Temperature boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. Though it’s one of the basic facts you probably learnt pretty early on. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). water always. When Water Starts Boiling The Temperature.
From exogdnxdi.blob.core.windows.net
How Long Does It Take For Boiling Water To Cool At Room Temperature at Linda French blog When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; At other elevations the melting point will change due to different ambient pressure. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at. When Water Starts Boiling The Temperature.
From dxoqzfoie.blob.core.windows.net
When Does Water Boil Temperature at Kathleen Conley blog When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. the melting point of water is. When Water Starts Boiling The Temperature.
From ar.inspiredpencil.com
Boiling Point Of Water For Kids When Water Starts Boiling The Temperature Though it’s one of the basic facts you probably learnt pretty early on. These changes are discussed further in a section below. water always boils at 100˚c, right? water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. the melting point of. When Water Starts Boiling The Temperature.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; water always boils at 100˚c, right? boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. the melting point. When Water Starts Boiling The Temperature.
From worldnewlive.com
At What Temperature Did The Water Start To Boil? Mastery Wiki When Water Starts Boiling The Temperature boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is. When Water Starts Boiling The Temperature.
From philschatz.com
Phase Change and Latent Heat · Physics When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. water boils at a lower temperature as you gain altitude. When Water Starts Boiling The Temperature.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Compound Interest When Water Starts Boiling The Temperature the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). At other elevations the melting point will change due to different ambient pressure. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar.. When Water Starts Boiling The Temperature.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? When Water Starts Boiling The Temperature the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. water always boils at 100˚c, right? water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Though it’s. When Water Starts Boiling The Temperature.
From aweseas.blogspot.com
Boiling Point Of Water Sea Level When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. water always boils at 100˚c, right? the melting point. When Water Starts Boiling The Temperature.
From www.chegg.com
Solved The temperature at which water starts to boil is When Water Starts Boiling The Temperature water always boils at 100˚c, right? the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Though it’s one of the basic facts you probably learnt pretty early on. At other elevations the melting point will change due to different ambient pressure. These changes. When Water Starts Boiling The Temperature.
From ar.inspiredpencil.com
Boiling Point Of Water Examples When Water Starts Boiling The Temperature the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). water always boils at 100˚c, right? the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. These changes are discussed further in. When Water Starts Boiling The Temperature.
From exoloidmh.blob.core.windows.net
How Do You Know When Water Is Boiling at Leona Gwin blog When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. boiling occurs when bubbles form within. When Water Starts Boiling The Temperature.
From indianapublicmedia.org
Cooking Question When Is Water Actually Boiling? A Moment of Science Indiana Public Media When Water Starts Boiling The Temperature These changes are discussed further in a section below. Though it’s one of the basic facts you probably learnt pretty early on. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). the standard boiling point, as defined by the iupac in 1982, is the temperature at. When Water Starts Boiling The Temperature.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin When Water Starts Boiling The Temperature water always boils at 100˚c, right? water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid;. When Water Starts Boiling The Temperature.
From mavink.com
Water Boiling Pressure Chart When Water Starts Boiling The Temperature These changes are discussed further in a section below. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the. When Water Starts Boiling The Temperature.
From dxoqzfoie.blob.core.windows.net
When Does Water Boil Temperature at Kathleen Conley blog When Water Starts Boiling The Temperature water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. At other elevations the melting point will change due to different ambient pressure. water always boils at 100˚c, right? the melting point of water is about 32 degrees fahrenheit (0 degrees celsius). When Water Starts Boiling The Temperature.
From www.chegg.com
Solved The temperature at which water starts to boil is When Water Starts Boiling The Temperature At other elevations the melting point will change due to different ambient pressure. Though it’s one of the basic facts you probably learnt pretty early on. These changes are discussed further in a section below. boiling occurs when bubbles form within a liquid, marking a change from a substance’s liquid or solid phase into a gas. water always. When Water Starts Boiling The Temperature.
From www.pinterest.com
Mastering Boiling Water Tips for Cooking at Altitude When Water Starts Boiling The Temperature the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Though it’s one of the basic facts you probably learnt pretty early on. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by. When Water Starts Boiling The Temperature.
From www.animalia-life.club
Boiling Point Of Water For Kids When Water Starts Boiling The Temperature water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). boiling occurs when bubbles form within a liquid, marking a change. When Water Starts Boiling The Temperature.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and Freezing Point PowerPoint When Water Starts Boiling The Temperature These changes are discussed further in a section below. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; At other elevations the melting point will change due to different ambient pressure. water always boils at 100˚c, right? boiling occurs when. When Water Starts Boiling The Temperature.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Vector Illustration of When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. At other elevations the melting point will. When Water Starts Boiling The Temperature.
From www.coursehero.com
[Solved] The temperature at which water starts to boil is called its boiling... Course Hero When Water Starts Boiling The Temperature boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; These changes are discussed further in a section below. the melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). the. When Water Starts Boiling The Temperature.