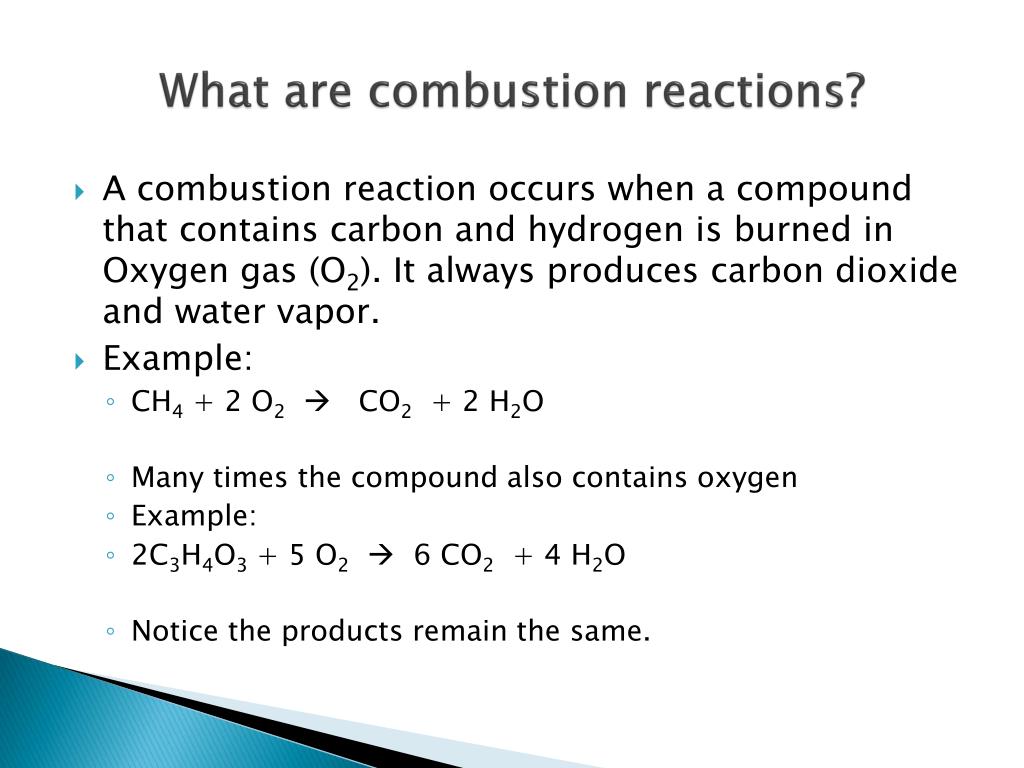
Combustion Definition Bbc . Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). A fuel is any compound which has stored energy. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. There are many examples of combustion.
from www.slideserve.com
Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. A fuel is any compound which has stored energy. There are many examples of combustion. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light.
PPT Combustion Reactions PowerPoint Presentation, free download ID
Combustion Definition Bbc Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is the name given to reactions where a fuel combines with oxygen. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. There are many examples of combustion. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. A fuel is any compound which has stored energy.
From www.slideshare.net
Combustion Reactions Slideshow123 Combustion Definition Bbc Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. A fuel is any compound which has stored energy. Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is burning a fuel. Combustion Definition Bbc.
From www.bbc.co.uk
Combustion what is it? BBC Bitesize Combustion Definition Bbc Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o),. Combustion Definition Bbc.
From www.thoughtco.com
Combustion Definition Chemistry Glossary Combustion Definition Bbc Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion is burning a fuel in oxygen, which gives out heat energy and is called. Combustion Definition Bbc.
From www.teachoo.com
Different Types of Combustion with Examples Teachoo Concepts Combustion Definition Bbc Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is the name given to reactions where a fuel combines with oxygen. A fuel is any compound which has stored energy. Learn about the combustion of. Combustion Definition Bbc.
From www.slideshare.net
Combustion Reactions Combustion Definition Bbc Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o),. Combustion Definition Bbc.
From www.bbc.co.uk
Combustion of fuels and the fire triangle test questions GCSE Combustion Definition Bbc Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry. Combustion Definition Bbc.
From www.slideshare.net
Heat of combustion of Various Alcohols Combustion Definition Bbc There are many examples of combustion. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide. Combustion Definition Bbc.
From www.javatpoint.com
Combustion Definition JavaTpoint Combustion Definition Bbc Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion is the name given to reactions where a fuel combines with oxygen. A fuel is any compound which has stored energy. Combustion is a reaction between a. Combustion Definition Bbc.
From www.slideserve.com
PPT Combustion Reaction PowerPoint Presentation, free download ID Combustion Definition Bbc Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. A fuel is any compound which has stored energy. There are many examples of combustion. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion requires fuel, oxygen, and an initial source of energy, usually heat,. Combustion Definition Bbc.
From www.youtube.com
Definition of Combustion for class 8 science. YouTube Combustion Definition Bbc Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. There are many examples of combustion. Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion is a reaction between a hydrocarbon fuel (e.g.,. Combustion Definition Bbc.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples Combustion Definition Bbc Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). A fuel is any compound which has stored energy. There are many examples of combustion. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat.. Combustion Definition Bbc.
From slideplayer.com
Types of Chemical Reactions ppt download Combustion Definition Bbc Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. A fuel is any compound which has stored energy. Combustion is the name given to. Combustion Definition Bbc.
From www.pinterest.co.uk
Combustion Reaction Definition and Examples Chemistry lessons Combustion Definition Bbc Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion is the name given to reactions where a fuel combines. Combustion Definition Bbc.
From sciencetrends.com
Combustion Reaction Examples And Definition Science Trends Combustion Definition Bbc There are many examples of combustion. Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion is a reaction between a hydrocarbon fuel. Combustion Definition Bbc.
From eleanor-has-tyler.blogspot.com
Enthalpy Change of Combustion EleanorhasTyler Combustion Definition Bbc There are many examples of combustion. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. A fuel is any compound which has stored energy. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is the chemical combination of a substance with oxygen, involving the production. Combustion Definition Bbc.
From www.slideserve.com
PPT How Can We Describe Chemical Reactions? PowerPoint Presentation Combustion Definition Bbc There are many examples of combustion. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion requires fuel, oxygen, and an initial source of. Combustion Definition Bbc.
From www.abcworksheet.com
What is Combustion Definition of Combustion Combustion Definition Bbc Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. There are many examples of combustion. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Learn about the combustion of fuels and the fire. Combustion Definition Bbc.
From www.tes.com
Combustion KS3 Teaching Resources Combustion Definition Bbc There are many examples of combustion. Combustion is the name given to reactions where a fuel combines with oxygen. A fuel is any compound which has stored energy. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen. Combustion Definition Bbc.
From www.bbc.co.uk
What is domestic energy guide for KS3 physics students BBC Bitesize Combustion Definition Bbc There are many examples of combustion. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). A fuel is any compound which has stored energy. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat.. Combustion Definition Bbc.
From engineerswikis.com
FUELS and COMBUSTION2 Combustion Definition Bbc There are many examples of combustion. Combustion is the name given to reactions where a fuel combines with oxygen. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h. Combustion Definition Bbc.
From www.slideserve.com
PPT Posters PowerPoint Presentation, free download ID3932001 Combustion Definition Bbc Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. A fuel is any compound which has stored energy. Combustion is the. Combustion Definition Bbc.
From www.slideshare.net
Combustion Reaction Combustion Definition Bbc Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. There are many examples of combustion. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion requires fuel, oxygen, and an initial source. Combustion Definition Bbc.
From slideshare.net
form1sciencechapter5part2 Combustion Definition Bbc Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion is the name given to reactions where a fuel combines with oxygen. Learn. Combustion Definition Bbc.
From www.thoughtco.com
Combustion Definition in Chemistry Combustion Definition Bbc Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). There are many examples of combustion. Combustion is the chemical combination of a substance with. Combustion Definition Bbc.
From www.shalom-education.com
Fuels and Combustion Shalom Education Combustion Definition Bbc Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and. Combustion Definition Bbc.
From www.slideshare.net
combustion Combustion Definition Bbc Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. There are many examples of combustion. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion is a reaction between. Combustion Definition Bbc.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID Combustion Definition Bbc Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is the name given to reactions where a fuel combines with oxygen. A fuel is any compound which has stored energy. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). There are many examples of combustion.. Combustion Definition Bbc.
From www.aquaportail.com
Combustion définition et explications Combustion Definition Bbc Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. A fuel is any compound which has stored energy. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion is. Combustion Definition Bbc.
From www.pinterest.co.kr
Combustion Reaction Definition, Characteristics & Examples Carbon Combustion Definition Bbc Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. There are many examples of combustion. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Learn about the combustion of fuels and the fire. Combustion Definition Bbc.
From www.slideshare.net
Classification of Chemical Reactions Combustion Definition Bbc Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A fuel is any compound which has stored energy. Combustion requires fuel, oxygen, and. Combustion Definition Bbc.
From www.grc.nasa.gov
Combustion Combustion Definition Bbc Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and. Combustion Definition Bbc.
From slideplayer.com
Types of Reactions There are literally millions of chemical reactions Combustion Definition Bbc Learn about the combustion of fuels and the fire triangle with bbc bitesize gcse chemistry (wjec). A fuel is any compound which has stored energy. There are many examples of combustion. Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and. Combustion Definition Bbc.
From gamesmartz.com
Combustion Definition & Image GameSmartz Combustion Definition Bbc Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is burning a fuel in oxygen, which gives out heat energy and is called an. Combustion is the name given to reactions where a fuel combines with oxygen. There are many examples of combustion. A fuel is any compound which has stored energy.. Combustion Definition Bbc.
From slideplayer.com
Standard Grade Chemistry Topic 5 ppt download Combustion Definition Bbc Combustion is the name given to reactions where a fuel combines with oxygen. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. There are many examples of combustion. Learn about the combustion of fuels and the. Combustion Definition Bbc.
From www.youtube.com
Chemical Reactions (3 of 11) Combustion Reactions, An Explanation YouTube Combustion Definition Bbc Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. A fuel is any compound which has stored energy. There are many examples of combustion. Combustion is the chemical combination of a substance with oxygen, involving the production of heat and light. Learn about the combustion of fuels and the fire triangle with bbc. Combustion Definition Bbc.