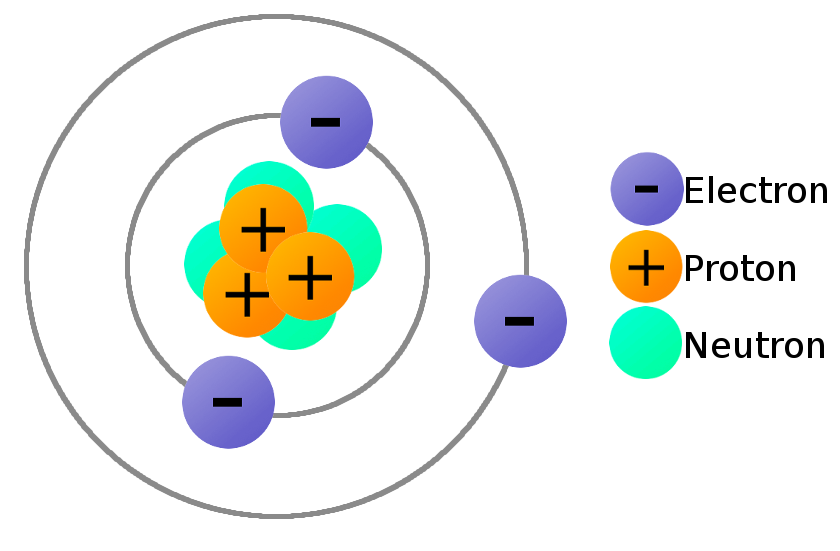
Lead Electrons Protons Neutrons . Atoms of an element that contain different numbers of neutrons are called isotopes. Neutral atoms have the same number of electrons and protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It has an atomic weight of 207.2 and a mass. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead.
from exoxpojqw.blob.core.windows.net
It has an atomic weight of 207.2 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Neutral atoms have the same number of electrons and protons. Atoms of an element that contain different numbers of neutrons are called isotopes.
Lead 214 Protons Neutrons Electrons at Heather Doss blog
Lead Electrons Protons Neutrons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. Atoms of an element that contain different numbers of neutrons are called isotopes. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It has an atomic weight of 207.2 and a mass. Neutral atoms have the same number of electrons and protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z.
From www.alamy.com
Lead (Pb). Diagram of the nuclear composition, electron configuration Lead Electrons Protons Neutrons Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Atoms of an element that contain different numbers of neutrons are called isotopes. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. 112 rows protons, neutrons and electrons of all elements. Lead Electrons Protons Neutrons.
From www.nuclear-power.com
Lead Atomic Number Atomic Mass Density of Lead Lead Electrons Protons Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Total number of protons in the nucleus is called the atomic number of the atom and is given. Lead Electrons Protons Neutrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Protons Neutrons Atoms of an element that contain different numbers of neutrons are called isotopes. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Neutral atoms have the same. Lead Electrons Protons Neutrons.
From quizlet.com
The diagram below shows some subatomic particles. A diagram, Quizlet Lead Electrons Protons Neutrons Neutral atoms have the same number of electrons and protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. 112 rows protons, neutrons and electrons of all elements are mentioned. Lead Electrons Protons Neutrons.
From dreamstime.com
Diagram Representation Of The Element Lead Stock Vector Image 59012885 Lead Electrons Protons Neutrons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It has an atomic weight of 207.2 and a mass. Lead is a chemical element. Lead Electrons Protons Neutrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Lead Electrons Protons Neutrons Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. It has an atomic weight of 207.2 and a mass. In this. Lead Electrons Protons Neutrons.
From room201csa.weebly.com
Atoms and Elements Científicos Matemáticos Lead Electrons Protons Neutrons Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. Atoms of an element that contain different numbers of neutrons are called isotopes. Neutral atoms have. Lead Electrons Protons Neutrons.
From www.expii.com
Neutrons — Structure & Properties Expii Lead Electrons Protons Neutrons Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. It has an atomic weight of 207.2 and a mass. Lead is a chemical element with atomic number 82. Lead Electrons Protons Neutrons.
From utedzz.blogspot.com
Periodic Table With Protons Electrons And Neutrons Periodic Table Lead Electrons Protons Neutrons Neutral atoms have the same number of electrons and protons. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Atoms of an element that contain different numbers of neutrons are called isotopes. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of. Lead Electrons Protons Neutrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Protons Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Neutral atoms have the same number of electrons and protons. It has an atomic weight of 207.2 and a mass. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus.. Lead Electrons Protons Neutrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Electrons Protons Neutrons Atoms of an element that contain different numbers of neutrons are called isotopes. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Neutral atoms have the same number of electrons and protons. Total number of protons in the nucleus. Lead Electrons Protons Neutrons.
From www.dreamstime.com
Lead stock illustration. Illustration of atomic, symbol 175796461 Lead Electrons Protons Neutrons It has an atomic weight of 207.2 and a mass. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Neutral atoms have the same number of electrons and protons.. Lead Electrons Protons Neutrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Protons Neutrons It has an atomic weight of 207.2 and a mass. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Atoms of an element that contain different numbers of neutrons are called. Lead Electrons Protons Neutrons.
From www.youtube.com
How to find Protons & Electrons for the Pb2+ and Pb4+ (Lead II and Lead Lead Electrons Protons Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Lead is a chemical element with atomic number 82 which. Lead Electrons Protons Neutrons.
From twobirdsfourhands.com
Lead Periodic Table Protons Two Birds Home Lead Electrons Protons Neutrons Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. Lead is a chemical element with atomic number 82 which means there are 82 protons. Lead Electrons Protons Neutrons.
From slideplayer.com
The Missing Manual of Historical Figures ppt download Lead Electrons Protons Neutrons It has an atomic weight of 207.2 and a mass. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list +. Lead Electrons Protons Neutrons.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Lead? Lead Electrons Protons Neutrons Neutral atoms have the same number of electrons and protons. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Atoms of an element that contain different numbers of. Lead Electrons Protons Neutrons.
From www.expii.com
Electrons — Structure & Properties Expii Lead Electrons Protons Neutrons Atoms of an element that contain different numbers of neutrons are called isotopes. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. It has an. Lead Electrons Protons Neutrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Protons Neutrons Neutral atoms have the same number of electrons and protons. It has an atomic weight of 207.2 and a mass. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Total number of protons in the nucleus is called the atomic number of the. Lead Electrons Protons Neutrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Pt (Platinum Lead Electrons Protons Neutrons Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Atoms of an element that contain different numbers of neutrons are called isotopes. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Neutral. Lead Electrons Protons Neutrons.
From www.britannica.com
lead summary Britannica Lead Electrons Protons Neutrons It has an atomic weight of 207.2 and a mass. Atoms of an element that contain different numbers of neutrons are called isotopes. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will. Lead Electrons Protons Neutrons.
From ar.inspiredpencil.com
Lead Atom Lead Electrons Protons Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Atoms of an element that contain different numbers of neutrons are called isotopes. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. In this video we’ll use. Lead Electrons Protons Neutrons.
From exoxpojqw.blob.core.windows.net
Lead 214 Protons Neutrons Electrons at Heather Doss blog Lead Electrons Protons Neutrons Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Neutral atoms have the same number of electrons and protons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol. Lead Electrons Protons Neutrons.
From www.broadlearnings.com
Atomic Structure Broad Learnings Lead Electrons Protons Neutrons Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Neutral atoms have the same number of electrons and protons. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead.. Lead Electrons Protons Neutrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Protons Neutrons Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms of an element that contain different numbers of neutrons are called isotopes. It has an atomic weight of 207.2 and a mass. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Lead is a chemical element with atomic number 82. Lead Electrons Protons Neutrons.
From www.teachoo.com
Neutron Discovery, Difference and more Teachoo Concepts Lead Electrons Protons Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. It has an atomic weight of 207.2 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms of an element that contain different numbers of neutrons are called isotopes. In this video we’ll use the periodic. Lead Electrons Protons Neutrons.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID151803 Lead Electrons Protons Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Neutral atoms have the same number of electrons and protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Lead is a chemical element with atomic. Lead Electrons Protons Neutrons.
From nl.wikihow.com
Het aantal neutronen, protonen en elektronen bepalen 6 stappen (met Lead Electrons Protons Neutrons Neutral atoms have the same number of electrons and protons. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. It has an atomic weight of 207.2 and a mass. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons. Lead Electrons Protons Neutrons.
From material-properties.org
Lead Protons Neutrons Electrons Electron Configuration Lead Electrons Protons Neutrons Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It has an atomic weight of 207.2 and a mass. Neutral atoms have the same number of electrons and protons. Lead is the 82nd element in the periodic table and has. Lead Electrons Protons Neutrons.
From mungfali.com
Protons Neutrons And Electrons Diagram Lead Electrons Protons Neutrons Atoms of an element that contain different numbers of neutrons are called isotopes. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. 112 rows protons,. Lead Electrons Protons Neutrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Protons Neutrons Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Atoms of an element that contain different numbers of neutrons are called isotopes. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Lead is a chemical element with. Lead Electrons Protons Neutrons.
From cabinet.matttroy.net
Lead Periodic Table Electrons Matttroy Lead Electrons Protons Neutrons Neutral atoms have the same number of electrons and protons. Atoms of an element that contain different numbers of neutrons are called isotopes. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Lead Electrons Protons Neutrons.
From www.sciencephoto.com
Lead, atomic structure Stock Image C013/1639 Science Photo Library Lead Electrons Protons Neutrons It has an atomic weight of 207.2 and a mass. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list +. Lead Electrons Protons Neutrons.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Tin? Lead Electrons Protons Neutrons Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Neutral atoms have the same number of electrons and protons. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. In this video we’ll. Lead Electrons Protons Neutrons.
From exoxpojqw.blob.core.windows.net
Lead 214 Protons Neutrons Electrons at Heather Doss blog Lead Electrons Protons Neutrons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element lead. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. 112 rows protons, neutrons and electrons of all. Lead Electrons Protons Neutrons.