Hco3 Vs H2Co3 . Moof's medical biochemistry video course: Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Find out the normal range, indications, pathophysiology, and clinical. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be.
from www.visionlearning.com
Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Moof's medical biochemistry video course: Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Find out the normal range, indications, pathophysiology, and clinical. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be.
Acids and Bases II Biology Visionlearning
Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Moof's medical biochemistry video course: Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Find out the normal range, indications, pathophysiology, and clinical. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and.
From slideplayer.com
THE RELATIONSHIP BETWEEN H2CO3* AND HCO3 ppt download Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Moof's medical biochemistry video course: Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Find. Hco3 Vs H2Co3.
From www.numerade.com
SOLVED In the reaction H2CO3+H2O⇋HCO3−+H3O+, the Bronsted acids are A Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Find. Hco3 Vs H2Co3.
From www.vrogue.co
Estructura De Lewis Del H2co3 Lios vrogue.co Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Moof's medical biochemistry video course: Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs.. Hco3 Vs H2Co3.
From www.numerade.com
SOLVED Which species are present in the solution between 14 mL and 22 Hco3 Vs H2Co3 Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Moof's medical biochemistry video course:. Hco3 Vs H2Co3.
From ar.inspiredpencil.com
Hco3 Lewis Structure Hco3 Vs H2Co3 Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Moof's medical biochemistry video course: Find out the normal range, indications, pathophysiology, and clinical. As carbon dioxide enters the blood, it combines with water to form carbonic acid,. Hco3 Vs H2Co3.
From www.chegg.com
Solved H2O+CO2↔H2CO3↔HCO3−+H+ ↓HCO−→↑H+→↑pH. ↑HCO3−→↓H+→↑pH. Hco3 Vs H2Co3 Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Moof's. Hco3 Vs H2Co3.
From www.numerade.com
SOLVED Carbon dioxide reacts with water in our blood to form carbonic Hco3 Vs H2Co3 Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. As carbon dioxide enters the blood, it combines with water. Hco3 Vs H2Co3.
From lambdageeks.com
15 Facts on HCl + H2CO3 What, How To Balance & FAQs LAMBDAGEEKS Hco3 Vs H2Co3 Moof's medical biochemistry video course: Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Carbonic acid can be considered to be a diprotic acid from which two series of. Hco3 Vs H2Co3.
From www.slideserve.com
PPT pH, acid neutralizing capacity & acid rain PowerPoint Hco3 Vs H2Co3 Moof's medical biochemistry video course: As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Find out the normal range, indications, pathophysiology, and clinical. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Bicarbonate and carbonic acid are both chemical compounds that play. Hco3 Vs H2Co3.
From marvelvietnam.com
base conjugate pairs in the following acid base reaction H2CO3 (aq Hco3 Vs H2Co3 Find out the normal range, indications, pathophysiology, and clinical. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Moof's medical biochemistry video course: As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Learn how acids and bases differ. Hco3 Vs H2Co3.
From www.slideserve.com
PPT K 1 = [HCO 3 ] [H + ] PowerPoint Presentation, free download Hco3 Vs H2Co3 Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Moof's medical biochemistry video course: Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Find out the normal range,. Hco3 Vs H2Co3.
From labpedia.net
Acidbase Balance Part 2 Metabolic acidosis, and Metabolic Hco3 Vs H2Co3 Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Find out the normal range, indications, pathophysiology, and clinical. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. As. Hco3 Vs H2Co3.
From www.numerade.com
SOLVED In the following reaction HCO3^ (aq) + H2O (aq) > H2CO3 Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Carbonic acid can be considered to. Hco3 Vs H2Co3.
From www.researchgate.net
10 Equivalence point pH for OHto H2O, CO3 2to HCO3, and HCO3to Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Moof's medical biochemistry video course: Find out the normal range, indications, pathophysiology, and clinical. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Learn how acids and bases differ only by the presence. Hco3 Vs H2Co3.
From www.numerade.com
SOLVED The pH of blood plasma is 7.40. The principal buffer system in Hco3 Vs H2Co3 Moof's medical biochemistry video course: Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Learn how acids and bases. Hco3 Vs H2Co3.
From www.numerade.com
SOLVED Consider the reaction below HCO3+ HCl > H2CO3 + Cl Which is Hco3 Vs H2Co3 Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Learn how acids and bases differ. Hco3 Vs H2Co3.
From www.chegg.com
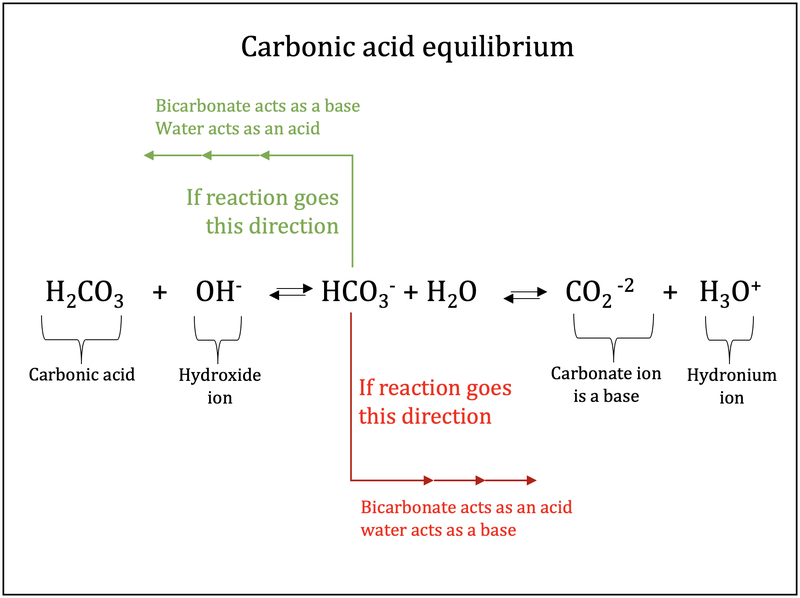
Consider the following reaction H2CO3+H2O⇌HCO3−+H3O+ Hco3 Vs H2Co3 Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Moof's medical biochemistry video course:. Hco3 Vs H2Co3.
From www.numerade.com
SOLVED What is the concentration of HCO3 in blood in mol/L (molarity Hco3 Vs H2Co3 Moof's medical biochemistry video course: Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Find out the normal range, indications, pathophysiology, and clinical. As carbon dioxide enters the blood, it combines with water to form carbonic. Hco3 Vs H2Co3.
From www.youtube.com
How to calculate the HCO3H2CO3 buffer ratio in blood YouTube Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Carbonic acid can be considered to. Hco3 Vs H2Co3.
From pubs.acs.org
H2CO3 Forms via HCO3− in Water The Journal of Physical Chemistry B Hco3 Vs H2Co3 Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Find out the normal range, indications, pathophysiology, and clinical. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules.. Hco3 Vs H2Co3.
From ar.inspiredpencil.com
Carbonic Acid Dissociation Hco3 Vs H2Co3 Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Moof's medical biochemistry video course: Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. As carbon dioxide enters. Hco3 Vs H2Co3.
From www.visionlearning.com
Acids and Bases II Biology Visionlearning Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Moof's medical. Hco3 Vs H2Co3.
From 90zavod.ru
Как получить h2co3 из co2 CCO2H2CO3CO2Na2CO3 Школьные Hco3 Vs H2Co3 Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Learn how acids and bases differ. Hco3 Vs H2Co3.
From www.studocu.com
H2CO3 Lewis Structure, Molecular Geometry, Hybridization, Polar or Hco3 Vs H2Co3 Moof's medical biochemistry video course: Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Find out the normal range, indications, pathophysiology, and clinical. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Carbonic acid can be considered to be a diprotic acid from which two series. Hco3 Vs H2Co3.
From www.researchgate.net
Interaction of fluxes of CO2, HCO3 − , H + , and CO3 2− with Hco3 Vs H2Co3 Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Find out the normal range,. Hco3 Vs H2Co3.
From www.youtube.com
How to Balance H2CO3 = H2O + CO2 of Carbonic acid) YouTube Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Moof's medical biochemistry video course: Find out the normal range, indications, pathophysiology, and clinical. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Co2 + h2o ⇌ h2co3. Hco3 Vs H2Co3.
From www.youtube.com
How to Balance NaHCO3 + HCl = NaCl + CO2 + H2O (Sodium Bicarbonate Plus Hco3 Vs H2Co3 Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Moof's medical biochemistry video course: Find out the normal range, indications, pathophysiology, and clinical. Co2 + h2o ⇌ h2co3 the predominant species are. Hco3 Vs H2Co3.
From www.aquaportail.com
Anhydride carbonique définition et explications Hco3 Vs H2Co3 As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Moof's. Hco3 Vs H2Co3.
From www.youtube.com
Type of Reaction for H2CO3 = H2O + CO2 YouTube Hco3 Vs H2Co3 Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Moof's medical biochemistry video course: As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Learn. Hco3 Vs H2Co3.
From wiener.me
Solved Reaction NaHCO3(aq) HCl(aq) → H2CO3(aq), 59 OFF Hco3 Vs H2Co3 Find out the normal range, indications, pathophysiology, and clinical. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. Carbonic acid can be considered to be a diprotic acid from which two series. Hco3 Vs H2Co3.
From www.youtube.com
Type of Reaction for NaHCO3 = Na2CO3 + H2O + CO2 YouTube Hco3 Vs H2Co3 Moof's medical biochemistry video course: Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Find out the normal range, indications, pathophysiology, and clinical. Co2 + h2o ⇌ h2co3 the. Hco3 Vs H2Co3.
From brainly.in
Calculate The Concentrations Of H+, HCO3, And CO3^2 In A 0.025M H2CO3 Hco3 Vs H2Co3 Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Find out the normal range, indications, pathophysiology, and clinical. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions. Hco3 Vs H2Co3.
From www.slideserve.com
PPT THE RELATIONSHIP BETWEEN H 2 CO 3 * AND HCO 3 PowerPoint Hco3 Vs H2Co3 Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Learn how acids and bases differ only by the presence. Hco3 Vs H2Co3.
From www.vrogue.co
Solved Water Hydrogen Ion Co2 H2o H2co3 H Hco3 Chegg vrogue.co Hco3 Vs H2Co3 Find out the normal range, indications, pathophysiology, and clinical. Bicarbonate and carbonic acid are both chemical compounds that play important roles in maintaining the ph balance in. Moof's medical biochemistry video course: Co2 + h2o ⇌ h2co3 the predominant species are simply loosely hydrated co2 molecules. Learn how acids and bases differ only by the presence or absence of a. Hco3 Vs H2Co3.
From www.numerade.com
SOLVED In the equation H2CO3 + H2O HCO3 + H3O+ Group of answer Hco3 Vs H2Co3 Learn how acids and bases differ only by the presence or absence of a proton and form conjugate pairs. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (h +) and. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be. Co2. Hco3 Vs H2Co3.