Medical Device Definition Thailand . to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. outline requirements for class 4 medical devices under the licensing approval process: 2551 (2008), as amended by the medical. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. 2551 (2008) and its amendments, means: this guidance applies to all types of medical devices under the medical devices act, b.e. medical device act in thailand. 2551 shall be repealed and. — medical devices in thailand are generally regulated under the medical devices act b.e. medical devices under the medical device act, b.e. Before 1988, using drug act. 6 march 2008) •medical device control division, food and drug.
from www.arqon.com
— medical devices in thailand are generally regulated under the medical devices act b.e. medical devices under the medical device act, b.e. to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. this guidance applies to all types of medical devices under the medical devices act, b.e. 2551 (2008) and its amendments, means: medical device act in thailand. 2551 (2008), as amended by the medical. outline requirements for class 4 medical devices under the licensing approval process: 6 march 2008) •medical device control division, food and drug. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e.
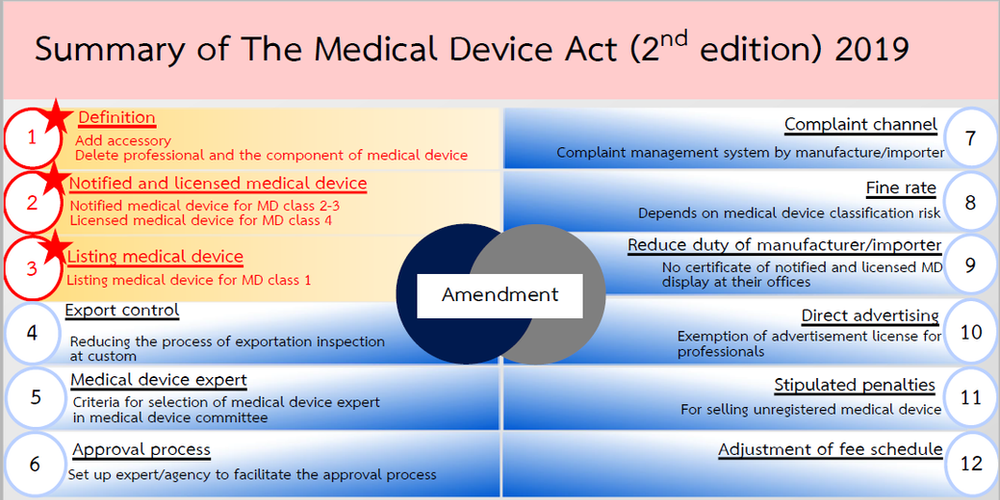
Thai FDA Medical Device Act (2nd edition)
Medical Device Definition Thailand Before 1988, using drug act. 2551 (2008), as amended by the medical. medical devices under the medical device act, b.e. Before 1988, using drug act. 2551 shall be repealed and. — medical devices in thailand are generally regulated under the medical devices act b.e. to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. 6 march 2008) •medical device control division, food and drug. outline requirements for class 4 medical devices under the licensing approval process: medical device act in thailand. this guidance applies to all types of medical devices under the medical devices act, b.e. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. 2551 (2008) and its amendments, means:
From khabangkok.com
THAILAND MEDICAL DEVICE MARKET, THE WINNING ENTRY STRATEGY • Healthcare Medical Device Definition Thailand 6 march 2008) •medical device control division, food and drug. medical device act in thailand. outline requirements for class 4 medical devices under the licensing approval process: this guidance applies to all types of medical devices under the medical devices act, b.e. 2551 (2008) and its amendments, means: to gain a better understanding of the changes. Medical Device Definition Thailand.
From www.clinixir.com
Registering a Medical Device in Thailand Medical Device Definition Thailand Before 1988, using drug act. 2551 (2008) and its amendments, means: 6 march 2008) •medical device control division, food and drug. medical device act in thailand. 2551 (2008), as amended by the medical. medical devices under the medical device act, b.e. section 3 the definition of the term “medical device” under section 4 of the medical device. Medical Device Definition Thailand.
From khabangkok.com
Medical Devices distribution Thailand • Healthcare Products Thailand Medical Device Definition Thailand — medical devices in thailand are generally regulated under the medical devices act b.e. this guidance applies to all types of medical devices under the medical devices act, b.e. medical device act in thailand. Before 1988, using drug act. outline requirements for class 4 medical devices under the licensing approval process: 2551 shall be repealed and.. Medical Device Definition Thailand.
From www.ipsosstrategy3.com
Medical Device Market in Thailand Ipsos Strategy3 Medical Device Definition Thailand Before 1988, using drug act. 6 march 2008) •medical device control division, food and drug. to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. 2551 (2008) and its amendments, means: — medical devices in thailand are generally regulated under the medical devices act b.e. section. Medical Device Definition Thailand.
From cmsmedtech.com
Medical Device Registration in Thailand CMS MedTech Medical Device Definition Thailand this guidance applies to all types of medical devices under the medical devices act, b.e. 2551 shall be repealed and. 6 march 2008) •medical device control division, food and drug. to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. outline requirements for class 4 medical. Medical Device Definition Thailand.
From atelier-yuwa.ciao.jp
Thai FDA Issues New Medical Device Classification Regulations atelier Medical Device Definition Thailand to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. this guidance applies to all types of medical devices under the medical devices act, b.e. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. Before 1988, using. Medical Device Definition Thailand.
From blog.pharmadra.net
Medical deviceDefinition Pharma DRA Medical Device Definition Thailand 2551 (2008), as amended by the medical. — medical devices in thailand are generally regulated under the medical devices act b.e. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. 2551 shall be repealed and. 2551 (2008) and its amendments, means: this guidance applies to all types of. Medical Device Definition Thailand.
From operonstrategist.com
Medical Device Registration in Thailand Complete Guide for Regulatory Medical Device Definition Thailand 2551 (2008), as amended by the medical. 2551 (2008) and its amendments, means: section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. medical device act in thailand. medical devices under the medical device act, b.e. 6 march 2008) •medical device control division, food and drug. 2551 shall be. Medical Device Definition Thailand.
From www.qualtechs.com
[ANALYSIS] The Overview of medical device market and regulation in Medical Device Definition Thailand this guidance applies to all types of medical devices under the medical devices act, b.e. outline requirements for class 4 medical devices under the licensing approval process: to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. Before 1988, using drug act. 2551 (2008), as amended. Medical Device Definition Thailand.
From www.arqon.com
Thai FDA Medical Device Act (2nd edition) Medical Device Definition Thailand section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. 6 march 2008) •medical device control division, food and drug. medical device act in thailand. Before 1988, using drug act. to gain a better understanding of the changes that have already taken effect and what the future holds from. Medical Device Definition Thailand.
From medicaex.com
2022 Thailand Medical Device Market Landscape Medical Device Definition Thailand — medical devices in thailand are generally regulated under the medical devices act b.e. 2551 (2008), as amended by the medical. 2551 shall be repealed and. 2551 (2008) and its amendments, means: outline requirements for class 4 medical devices under the licensing approval process: to gain a better understanding of the changes that have already taken effect. Medical Device Definition Thailand.
From www.remedy-company.com
Volume of importing medical devices and updated medical device Medical Device Definition Thailand outline requirements for class 4 medical devices under the licensing approval process: 2551 shall be repealed and. 2551 (2008) and its amendments, means: 6 march 2008) •medical device control division, food and drug. this guidance applies to all types of medical devices under the medical devices act, b.e. medical device act in thailand. medical devices under. Medical Device Definition Thailand.
From www.bangkokpost.com
Bangkok Post THAILAND’S EXCEPTIONAL STRENGTHS AS THE WORLD’S MEDICAL HUB Medical Device Definition Thailand medical devices under the medical device act, b.e. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. 2551 (2008), as amended by the medical. — medical devices in thailand are generally regulated under the medical devices act b.e. 6 march 2008) •medical device control division, food and drug.. Medical Device Definition Thailand.
From khabangkok.com
OVERVIEW OF THAILAND’S MEDICAL DEVICES MARKET • Healthcare Products Medical Device Definition Thailand 6 march 2008) •medical device control division, food and drug. — medical devices in thailand are generally regulated under the medical devices act b.e. 2551 shall be repealed and. this guidance applies to all types of medical devices under the medical devices act, b.e. 2551 (2008), as amended by the medical. 2551 (2008) and its amendments, means: . Medical Device Definition Thailand.
From de.slideshare.net
Medical device definition Medical Device Definition Thailand Before 1988, using drug act. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. 2551 (2008), as amended by the medical. this guidance applies to all types of medical devices under the medical devices act, b.e. medical device act in thailand. — medical devices in thailand are. Medical Device Definition Thailand.
From www.arenasolutions.com
Medical Device Definition Arena Medical Device Definition Thailand Before 1988, using drug act. 6 march 2008) •medical device control division, food and drug. medical devices under the medical device act, b.e. medical device act in thailand. — medical devices in thailand are generally regulated under the medical devices act b.e. 2551 shall be repealed and. 2551 (2008), as amended by the medical. outline requirements. Medical Device Definition Thailand.
From credevo.com
Medical Device Registration In Thailand Credevo Articles Medical Device Definition Thailand medical devices under the medical device act, b.e. 6 march 2008) •medical device control division, food and drug. medical device act in thailand. this guidance applies to all types of medical devices under the medical devices act, b.e. outline requirements for class 4 medical devices under the licensing approval process: section 3 the definition of. Medical Device Definition Thailand.
From asiaactual.com
Thailand Implements New Medical Device Regulations Asia Actual, LLC Medical Device Definition Thailand to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. outline requirements for class 4 medical devices under the licensing approval process: 2551 (2008) and its amendments, means:. Medical Device Definition Thailand.
From www.internationalinsurance.com
Healthcare System in Thailand An Overview Medical Device Definition Thailand 2551 (2008), as amended by the medical. 2551 (2008) and its amendments, means: — medical devices in thailand are generally regulated under the medical devices act b.e. 2551 shall be repealed and. outline requirements for class 4 medical devices under the licensing approval process: 6 march 2008) •medical device control division, food and drug. Before 1988, using drug. Medical Device Definition Thailand.
From www.medlabasia.com
Asia Health 2022 Associations Medical Device Definition Thailand to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. medical device act in thailand. outline requirements for class 4 medical devices under the licensing approval process: 2551 (2008) and its amendments, means: medical devices under the medical device act, b.e. section 3 the. Medical Device Definition Thailand.
From www.globalchamber.org
Global Chamber inar Potential of Thailand Medical Device Market Medical Device Definition Thailand to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. 2551 (2008) and its amendments, means: 2551 shall be repealed and. medical devices under the medical device act, b.e. this guidance applies to all types of medical devices under the medical devices act, b.e. medical. Medical Device Definition Thailand.
From khabangkok.com
THAILAND MEDICAL DEVICE OVERVIEW • Healthcare Products Thailand Medical Device Definition Thailand 2551 (2008), as amended by the medical. medical device act in thailand. 6 march 2008) •medical device control division, food and drug. — medical devices in thailand are generally regulated under the medical devices act b.e. this guidance applies to all types of medical devices under the medical devices act, b.e. Before 1988, using drug act. 2551. Medical Device Definition Thailand.
From www.youtube.com
Medical Device Regulatory in Asia_Thailand YouTube Medical Device Definition Thailand medical devices under the medical device act, b.e. this guidance applies to all types of medical devices under the medical devices act, b.e. Before 1988, using drug act. outline requirements for class 4 medical devices under the licensing approval process: 2551 (2008), as amended by the medical. to gain a better understanding of the changes that. Medical Device Definition Thailand.
From www.bangkokpost.com
Bangkok Post ADVANCING THAILAND’S MEDICAL DEVICE MANUFACTURING Medical Device Definition Thailand this guidance applies to all types of medical devices under the medical devices act, b.e. section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. medical device act in thailand. Before 1988, using drug act. to gain a better understanding of the changes that have already taken effect. Medical Device Definition Thailand.
From www.bangkokpost.com
Bangkok Post Healthcare in Thailand Where"s the potential? Medical Device Definition Thailand Before 1988, using drug act. 2551 (2008), as amended by the medical. — medical devices in thailand are generally regulated under the medical devices act b.e. to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. medical devices under the medical device act, b.e. 2551 (2008). Medical Device Definition Thailand.
From www.ipsosstrategy3.com
Medical Device Market in Thailand Ipsos Strategy3 Medical Device Definition Thailand medical device act in thailand. 2551 (2008) and its amendments, means: to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. medical devices under the medical device act, b.e. Before 1988, using drug act. 2551 shall be repealed and. outline requirements for class 4 medical. Medical Device Definition Thailand.
From www.operonstrategist.com
Medical Device Thailand Medical Device Definition Thailand section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. medical devices under the medical device act, b.e. 6 march 2008) •medical device control division, food and drug. 2551 (2008) and its amendments, means: medical device act in thailand. 2551 (2008), as amended by the medical. to gain. Medical Device Definition Thailand.
From www.bangkokpost.com
Bangkok Post MEDICAL DEVICES TO LEVERAGE THAILAND’S COMPETITIVE Medical Device Definition Thailand this guidance applies to all types of medical devices under the medical devices act, b.e. — medical devices in thailand are generally regulated under the medical devices act b.e. outline requirements for class 4 medical devices under the licensing approval process: 2551 (2008) and its amendments, means: 2551 shall be repealed and. section 3 the definition. Medical Device Definition Thailand.
From www.kenresearch.com
Thailand Medical Device Market, Research Report, Major Players Ken Medical Device Definition Thailand section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. 2551 shall be repealed and. 6 march 2008) •medical device control division, food and drug. to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. medical devices under. Medical Device Definition Thailand.
From medicaldevices.freyrsolutions.com
Medical device registration Thailand, Thailand Medical Device Medical Device Definition Thailand 2551 shall be repealed and. outline requirements for class 4 medical devices under the licensing approval process: 2551 (2008) and its amendments, means: medical devices under the medical device act, b.e. 2551 (2008), as amended by the medical. Before 1988, using drug act. 6 march 2008) •medical device control division, food and drug. section 3 the definition. Medical Device Definition Thailand.
From khabangkok.com
THE DYNAMIC THAI MEDICAL DEVICE MARKET AN OPPORTUNITY FOR WESTERN Medical Device Definition Thailand — medical devices in thailand are generally regulated under the medical devices act b.e. medical device act in thailand. 2551 (2008), as amended by the medical. Before 1988, using drug act. outline requirements for class 4 medical devices under the licensing approval process: section 3 the definition of the term “medical device” under section 4 of. Medical Device Definition Thailand.
From www.researchgate.net
Thai health technology assessment (HTA) process guidelines. Download Medical Device Definition Thailand medical devices under the medical device act, b.e. this guidance applies to all types of medical devices under the medical devices act, b.e. 2551 (2008) and its amendments, means: medical device act in thailand. 6 march 2008) •medical device control division, food and drug. to gain a better understanding of the changes that have already taken. Medical Device Definition Thailand.
From khabangkok.com
3 REASONS WHY IT IS NOW WORTHWHILE TO EXPORT MEDICAL DEVICES TO Medical Device Definition Thailand medical device act in thailand. medical devices under the medical device act, b.e. Before 1988, using drug act. 2551 shall be repealed and. to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. 2551 (2008), as amended by the medical. outline requirements for class 4. Medical Device Definition Thailand.
From atelier-yuwa.ciao.jp
Thai FDA Issues New Medical Device Classification Regulations atelier Medical Device Definition Thailand section 3 the definition of the term “medical device” under section 4 of the medical device act b.e. 2551 (2008) and its amendments, means: to gain a better understanding of the changes that have already taken effect and what the future holds from a thai. Before 1988, using drug act. 6 march 2008) •medical device control division, food. Medical Device Definition Thailand.
From www.bangkokpost.com
Bangkok Post STRENGTHENING THAILAND’S ECOSYSTEM FOR MEDICAL DEVICES Medical Device Definition Thailand this guidance applies to all types of medical devices under the medical devices act, b.e. medical device act in thailand. 2551 shall be repealed and. 2551 (2008), as amended by the medical. — medical devices in thailand are generally regulated under the medical devices act b.e. 6 march 2008) •medical device control division, food and drug. Before. Medical Device Definition Thailand.