Pressure Definition Chemistry Quizlet . Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Define the property of pressure; Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. We know that pressure and volume are inversely related; We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Pressure is decreasing (from 2.44 atm to 1.93. Describe the operation of common tools for measuring gas pressure. As one decreases, the other increases. Define and convert among the units of pressure measurements. Define and convert among the units of pressure measurements; Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more.
from www.youtube.com
We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Pressure is decreasing (from 2.44 atm to 1.93. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. We know that pressure and volume are inversely related; Define and convert among the units of pressure measurements; Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Define the property of pressure; As one decreases, the other increases. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more.
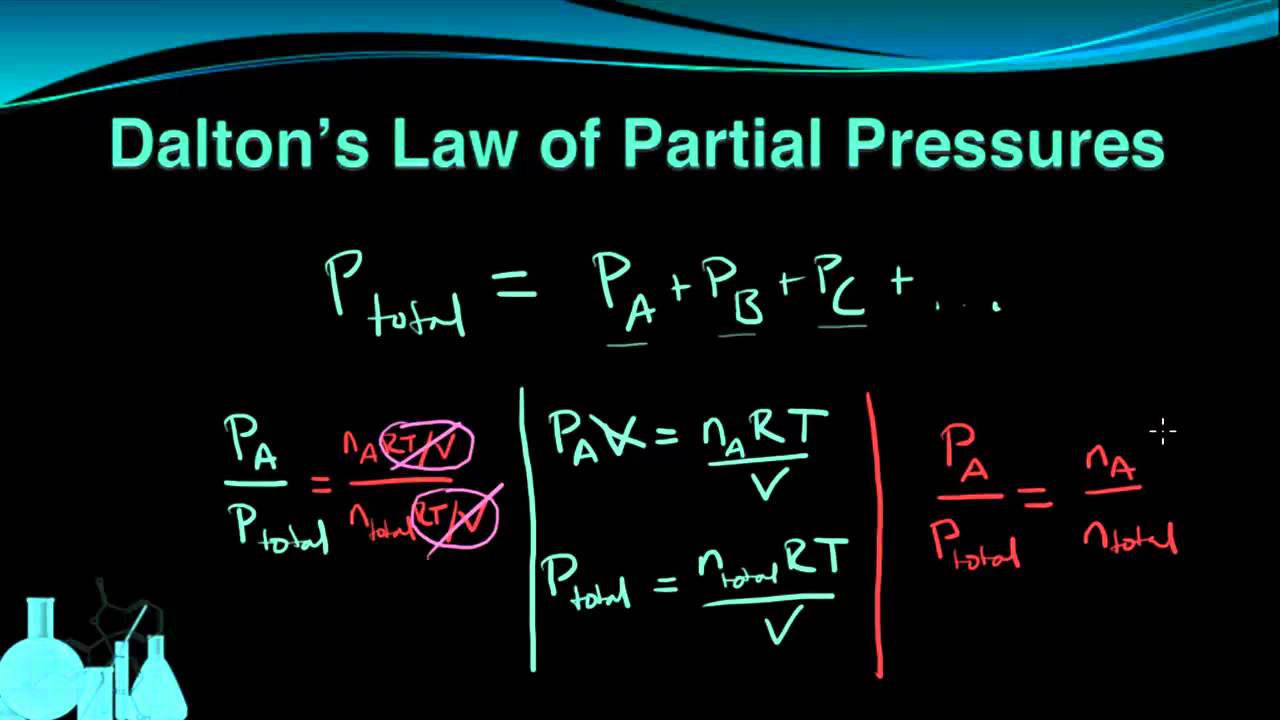
Chemistry 7.6 Dalton's Law of Partial Pressures YouTube
Pressure Definition Chemistry Quizlet As one decreases, the other increases. Describe the operation of common tools for measuring gas pressure. Define and convert among the units of pressure measurements; Define and convert among the units of pressure measurements. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. We know that pressure and volume are inversely related; Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Define the property of pressure; Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Pressure is decreasing (from 2.44 atm to 1.93. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. As one decreases, the other increases. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,.
From www.geeksforgeeks.org
Vapour Pressure Definition, Raoult's Law, Formula & FAQs Pressure Definition Chemistry Quizlet Pressure is decreasing (from 2.44 atm to 1.93. Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. Define and convert among the units of pressure measurements; Define the property of pressure;. Pressure Definition Chemistry Quizlet.
From www.slideserve.com
PPT Chemistry 231 PowerPoint Presentation, free download ID816731 Pressure Definition Chemistry Quizlet Define and convert among the units of pressure measurements; Pressure is decreasing (from 2.44 atm to 1.93. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. Define and convert among the units of pressure measurements. Describe the operation of common tools for measuring gas pressure. Dalton’s law, or the law of. Pressure Definition Chemistry Quizlet.
From www.youtube.com
Vapour Pressure & Raoults Law Chemistry Class 12 IIT JEE Main Pressure Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Define and convert among the units of pressure measurements; Study with quizlet and memorize flashcards. Pressure Definition Chemistry Quizlet.
From learningdbblake.z21.web.core.windows.net
Vapor Pressure And Boiling Worksheets Pressure Definition Chemistry Quizlet Define the property of pressure; Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Define and convert among the units of pressure measurements; We know that pressure and volume are inversely related; Describe the operation of common tools for measuring gas pressure. Define and convert among the units of. Pressure Definition Chemistry Quizlet.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Pressure Definition Chemistry Quizlet Describe the operation of common tools for measuring gas pressure. Define and convert among the units of pressure measurements. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Study with quizlet and. Pressure Definition Chemistry Quizlet.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Pressure Definition Chemistry Quizlet Describe the operation of common tools for measuring gas pressure. Pressure is decreasing (from 2.44 atm to 1.93. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Define and convert among the units of pressure measurements; As one decreases, the other increases. Study with. Pressure Definition Chemistry Quizlet.
From sciencenotes.org
Pressure Definition in Science Pressure Definition Chemistry Quizlet As one decreases, the other increases. Pressure is decreasing (from 2.44 atm to 1.93. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to.. Pressure Definition Chemistry Quizlet.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Pressure Definition Chemistry Quizlet As one decreases, the other increases. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. Pressure is decreasing (from 2.44 atm to 1.93. Describe the operation of common tools for measuring gas pressure. We. Pressure Definition Chemistry Quizlet.
From www.pinterest.com
Vapor Pressure Easy Science Chemistry education, Learn physics Pressure Definition Chemistry Quizlet Define and convert among the units of pressure measurements. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Define the property of pressure; We know that pressure and volume are inversely related; Describe the operation of common tools for measuring gas pressure. Study. Pressure Definition Chemistry Quizlet.
From www.youtube.com
Chemistry 7.6 Dalton's Law of Partial Pressures YouTube Pressure Definition Chemistry Quizlet Define and convert among the units of pressure measurements; Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Describe the operation of common tools for measuring gas pressure. As one decreases, the other increases. Study with quizlet and memorize flashcards containing terms like. Pressure Definition Chemistry Quizlet.
From www.sliderbase.com
Measurement of Pressure and Temperature Presentation Chemistry Pressure Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Define and convert among the units of pressure measurements; Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. We find that temperature and pressure are linearly related, and if the temperature. Pressure Definition Chemistry Quizlet.
From whatsinsight.org
Atmospheric Pressure Definition & RealLife Examples What's Insight Pressure Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Define and convert among the units of pressure measurements; As one decreases, the other increases. Pressure is decreasing (from 2.44 atm to 1.93. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,.. Pressure Definition Chemistry Quizlet.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Pressure Definition Chemistry Quizlet Pressure is decreasing (from 2.44 atm to 1.93. Define the property of pressure; As one decreases, the other increases. Define and convert among the units of pressure measurements; Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Define and convert among the units. Pressure Definition Chemistry Quizlet.
From www.thoughtco.com
Pressure Definition and Examples (Science) Pressure Definition Chemistry Quizlet Define the property of pressure; Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. We know that pressure and volume are inversely related; Dalton’s law, or the law of partial pressures, states that the. Pressure Definition Chemistry Quizlet.
From www.pinterest.com
Partial Pressure Easy Science Easy science, Chemical changes, Pressure Pressure Definition Chemistry Quizlet We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. Pressure is decreasing (from 2.44 atm to 1.93. Define the property of pressure; We know that pressure. Pressure Definition Chemistry Quizlet.
From www.youtube.com
Define the term pressure. 8 FORCE AND PRESSURE PHYSICS ICSE Pressure Definition Chemistry Quizlet We know that pressure and volume are inversely related; Pressure is decreasing (from 2.44 atm to 1.93. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin. Pressure Definition Chemistry Quizlet.
From www.logiota.com
Dalton's Law of Partial Pressure States of Matter Physical Pressure Definition Chemistry Quizlet Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. As one decreases, the other increases. Define and convert among the units of pressure measurements; Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. We. Pressure Definition Chemistry Quizlet.
From greenishco.com
Pressure Definition Greenish Company Pressure Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Pressure is decreasing (from 2.44 atm to 1.93. Define and convert among the units of pressure measurements. Define the property of pressure; Describe the operation of common tools for measuring gas pressure. Define and convert among the units of pressure. Pressure Definition Chemistry Quizlet.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID2214653 Pressure Definition Chemistry Quizlet Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Define the property of pressure; We know that pressure and volume are inversely related; Define and convert among the units of pressure measurements; Study with quizlet and memorize flashcards containing terms like pressure definition,. Pressure Definition Chemistry Quizlet.
From www.slideserve.com
PPT GASES PowerPoint Presentation, free download ID7010102 Pressure Definition Chemistry Quizlet Define and convert among the units of pressure measurements. As one decreases, the other increases. Pressure is decreasing (from 2.44 atm to 1.93. Define the property of pressure; Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular. Pressure Definition Chemistry Quizlet.
From brunofuga.adv.br
Vapor Pressure Definition And How To Calculate It, 51 OFF Pressure Definition Chemistry Quizlet Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. As one decreases, the other increases. Define and convert among the units of pressure measurements; We know that pressure and volume are inversely related; We find that temperature and pressure are linearly related, and. Pressure Definition Chemistry Quizlet.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID6918783 Pressure Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. Pressure is decreasing (from 2.44 atm to 1.93. We know that pressure and volume are inversely related; We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Define. Pressure Definition Chemistry Quizlet.
From www.worksheetsplanet.com
What is Pressure Definition of Pressure Pressure Definition Chemistry Quizlet Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Define and convert among the units of pressure measurements. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. As one decreases, the other increases. We find. Pressure Definition Chemistry Quizlet.
From quizlet.com
Pressure, Density and Moments Diagram Quizlet Pressure Definition Chemistry Quizlet We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Pressure is decreasing (from 2.44 atm to 1.93. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Define. Pressure Definition Chemistry Quizlet.
From www.youtube.com
Colligative properties Vapor pressure lowering YouTube Pressure Definition Chemistry Quizlet We know that pressure and volume are inversely related; Define the property of pressure; Describe the operation of common tools for measuring gas pressure. Define and convert among the units of pressure measurements; Define and convert among the units of pressure measurements. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more.. Pressure Definition Chemistry Quizlet.
From www.chemistrylearner.com
Osmotic Pressure Definition, Formula, and Examples Pressure Definition Chemistry Quizlet Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. Define the property of pressure; We know that pressure and volume are inversely related; Define and convert among the units of pressure measurements; As one decreases, the other increases. Study with quizlet and memorize. Pressure Definition Chemistry Quizlet.
From chem.libretexts.org
10.2 Pressure Chemistry LibreTexts Pressure Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional.. Pressure Definition Chemistry Quizlet.
From worksheetlibrarysteins.z19.web.core.windows.net
Dalton's Law Of Partial Pressure Worksheet Pressure Definition Chemistry Quizlet Define and convert among the units of pressure measurements. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. As one decreases, the other increases. Describe the operation of common tools for measuring gas pressure. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin. Pressure Definition Chemistry Quizlet.
From eduinput.com
PressureDefinition, Formula, Types, And Applications Pressure Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. We know that pressure and volume are inversely related; Define the property of pressure; Study with quizlet and memorize flashcards containing terms like pressure, formula of pressure, what two properties can we change to. We find that temperature and pressure are linearly. Pressure Definition Chemistry Quizlet.
From www.youtube.com
Types of pressure, definition of absolute and gauge, vacuum and Pressure Definition Chemistry Quizlet We know that pressure and volume are inversely related; Define the property of pressure; Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. As one decreases,. Pressure Definition Chemistry Quizlet.
From www.youtube.com
osmosis and osmotic pressure solutions solutions class 12 chemistry Pressure Definition Chemistry Quizlet Pressure is decreasing (from 2.44 atm to 1.93. Describe the operation of common tools for measuring gas pressure. Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. Study with quizlet and memorize flashcards containing terms like pressure definition, molecular motion and density with temperature,. Define and convert among the units of. Pressure Definition Chemistry Quizlet.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4496385 Pressure Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. As one decreases, the other increases. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Define the property of pressure; Define and convert among the units. Pressure Definition Chemistry Quizlet.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples Pressure Definition Chemistry Quizlet We know that pressure and volume are inversely related; As one decreases, the other increases. Define and convert among the units of pressure measurements; Study with quizlet and memorize flashcards containing terms like volume, number of particles, temp in k and more. Describe the operation of common tools for measuring gas pressure. Study with quizlet and memorize flashcards containing terms. Pressure Definition Chemistry Quizlet.
From pediaa.com
Difference Between Partial Pressure and Vapor Pressure Definition Pressure Definition Chemistry Quizlet We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the. As one decreases, the other increases. Study with quizlet. Pressure Definition Chemistry Quizlet.
From gamesmartz.com
Atmospheric Pressure Definition & Image GameSmartz Pressure Definition Chemistry Quizlet Define and convert among the units of pressure measurements; We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. Define and convert among the units of pressure measurements. Define the property of pressure; We know that pressure and volume are inversely related; As one decreases,. Pressure Definition Chemistry Quizlet.