What Are Three Ways To Increase The Pressure Of A Gas . A decrease in container volume increases gas pressure. An increase in temperature of a gas in a rigid container increases the pressure. an increase in the number of gas molecules, while container volume stays constant, increases pressure. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. A calculate the force exerted by the book and then compute the area that is in contact with a surface. Boyle's law tells us that. increasing the temperature of a gas increases the pressure and the energy of the gas particles.
from masterconceptsinchemistry.com
pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. A decrease in container volume increases gas pressure. Boyle's law tells us that. increasing the temperature of a gas increases the pressure and the energy of the gas particles. An increase in temperature of a gas in a rigid container increases the pressure. an increase in the number of gas molecules, while container volume stays constant, increases pressure. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. A calculate the force exerted by the book and then compute the area that is in contact with a surface.
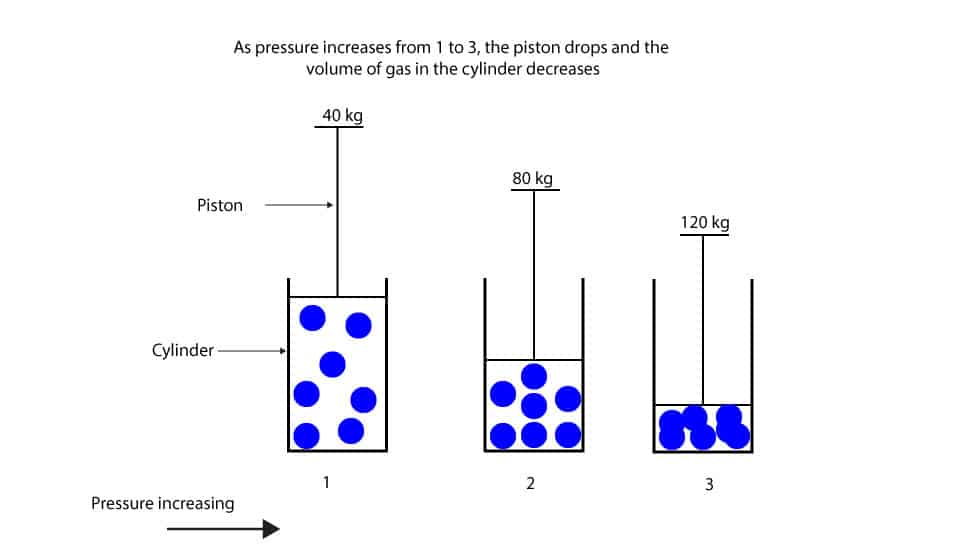
As pressure increase the volume of gas decrease
What Are Three Ways To Increase The Pressure Of A Gas The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. A decrease in container volume increases gas pressure. increasing the temperature of a gas increases the pressure and the energy of the gas particles. An increase in temperature of a gas in a rigid container increases the pressure. Boyle's law tells us that. an increase in the number of gas molecules, while container volume stays constant, increases pressure. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. A calculate the force exerted by the book and then compute the area that is in contact with a surface.
From www.slideserve.com
PPT Chapter 10 Gases PowerPoint Presentation, free download ID670308 What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. A calculate the force exerted by the book and then compute the area that is in contact with a surface. an increase in the number of gas molecules, while container volume stays constant, increases pressure. A decrease in container volume increases gas pressure. Boyle's law. What Are Three Ways To Increase The Pressure Of A Gas.
From www.nagwa.com
Question Video Identifying the Relationship between the Pressure and What Are Three Ways To Increase The Pressure Of A Gas Boyle's law tells us that. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. A decrease in container volume increases gas pressure. The three fundamental gas laws discover the relationship of pressure, temperature,. What Are Three Ways To Increase The Pressure Of A Gas.
From boisestate.pressbooks.pub
8.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas What Are Three Ways To Increase The Pressure Of A Gas Boyle's law tells us that. A calculate the force exerted by the book and then compute the area that is in contact with a surface. A decrease in container volume increases gas pressure. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. learn about and revise particle motion, gas pressure and the. What Are Three Ways To Increase The Pressure Of A Gas.
From masterconceptsinchemistry.com
As pressure increase the volume of gas decrease What Are Three Ways To Increase The Pressure Of A Gas The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. increasing the temperature of a gas increases the pressure and the energy of the gas particles. an increase in the number of gas molecules, while container volume stays constant, increases pressure. pressure is caused by the collisions between the atoms of. What Are Three Ways To Increase The Pressure Of A Gas.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo What Are Three Ways To Increase The Pressure Of A Gas increasing the temperature of a gas increases the pressure and the energy of the gas particles. an increase in the number of gas molecules, while container volume stays constant, increases pressure. A decrease in container volume increases gas pressure. A calculate the force exerted by the book and then compute the area that is in contact with a. What Are Three Ways To Increase The Pressure Of A Gas.
From www.youtube.com
Calculating Gas Pressure Pressure in the Gas Laws CLEAR & SIMPLE What Are Three Ways To Increase The Pressure Of A Gas an increase in the number of gas molecules, while container volume stays constant, increases pressure. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. A calculate the force exerted by the book and then compute the area that is in contact with a surface. Boyle's law tells us. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download What Are Three Ways To Increase The Pressure Of A Gas The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. An increase in temperature of a gas in a rigid container increases the pressure. A decrease in container volume increases gas pressure. Boyle's law. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT STATES OF MATTER PowerPoint Presentation, free download ID3230726 What Are Three Ways To Increase The Pressure Of A Gas learn about and revise particle motion, gas pressure and the relationship between pressure and volume. A decrease in container volume increases gas pressure. A calculate the force exerted by the book and then compute the area that is in contact with a surface. pressure is caused by the collisions between the atoms of gas and walls of the. What Are Three Ways To Increase The Pressure Of A Gas.
From www.numerade.com
SOLVEDA classmate correctly states that there are three ways to What Are Three Ways To Increase The Pressure Of A Gas an increase in the number of gas molecules, while container volume stays constant, increases pressure. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. A decrease in container volume increases gas pressure. Boyle's law tells us that. An increase in temperature of a gas in a rigid container increases the pressure. . What Are Three Ways To Increase The Pressure Of A Gas.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps What Are Three Ways To Increase The Pressure Of A Gas The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. An increase in temperature of a gas in a rigid container increases the pressure. Boyle's law tells us that. A decrease in container volume increases gas pressure. an increase in the number of gas molecules, while container volume stays constant, increases pressure. . What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Chemistry 14.1 PowerPoint Presentation, free download ID4874125 What Are Three Ways To Increase The Pressure Of A Gas increasing the temperature of a gas increases the pressure and the energy of the gas particles. An increase in temperature of a gas in a rigid container increases the pressure. A decrease in container volume increases gas pressure. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. an increase in the. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Compressibility PowerPoint Presentation, free download ID4200029 What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. A decrease in container volume increases gas pressure. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. an increase in the number. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Chapter 11 Gases PowerPoint Presentation, free download ID64297 What Are Three Ways To Increase The Pressure Of A Gas A calculate the force exerted by the book and then compute the area that is in contact with a surface. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. A decrease in container volume increases gas pressure. learn about and revise particle motion, gas pressure and the relationship between pressure and volume.. What Are Three Ways To Increase The Pressure Of A Gas.
From solvedlib.com
3 Does the pressure of a gas in a container increase … SolvedLib What Are Three Ways To Increase The Pressure Of A Gas pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. A calculate the force exerted by the book and then compute the area that is in contact with a surface. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. Boyle's law tells us. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT HSC CHEMISTRY CORE TOPIC 2 PowerPoint Presentation, free download What Are Three Ways To Increase The Pressure Of A Gas A decrease in container volume increases gas pressure. Boyle's law tells us that. A calculate the force exerted by the book and then compute the area that is in contact with a surface. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. pressure is caused by the collisions between the atoms of. What Are Three Ways To Increase The Pressure Of A Gas.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. A decrease in container volume increases gas pressure. an increase in the number of gas molecules, while container volume stays constant, increases pressure. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. pressure is caused by the. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Dalton’s Law The total pressure of a mixture of gases equals the What Are Three Ways To Increase The Pressure Of A Gas Boyle's law tells us that. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. A calculate the force exerted by the book and then compute the area that is in contact with a. What Are Three Ways To Increase The Pressure Of A Gas.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube What Are Three Ways To Increase The Pressure Of A Gas increasing the temperature of a gas increases the pressure and the energy of the gas particles. A decrease in container volume increases gas pressure. an increase in the number of gas molecules, while container volume stays constant, increases pressure. pressure is caused by the collisions between the atoms of gas and walls of the container as those. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Gas MixturesPartial Pressure PowerPoint Presentation, free What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. Boyle's law tells us that. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. increasing the temperature of a gas increases the pressure and the energy of the gas particles. The three fundamental gas laws discover the relationship. What Are Three Ways To Increase The Pressure Of A Gas.
From www.drawittoknowit.com
Physiology Glossary Gas Exchange Advanced Draw It to Know It What Are Three Ways To Increase The Pressure Of A Gas pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. An increase in temperature of a gas in a rigid container increases the pressure. A decrease in container volume increases gas pressure. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. an. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT GAS EXCHANGE AND GAS TRANSFER PowerPoint Presentation, free What Are Three Ways To Increase The Pressure Of A Gas pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. An increase in temperature of a gas in a rigid container increases the pressure. increasing the temperature of a gas increases the pressure and the energy of the gas particles. learn about and revise particle motion, gas pressure. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Transport of gases PowerPoint Presentation, free download ID What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. A calculate the force exerted by the book and then compute the area that is in contact with a surface. A decrease in container volume increases gas pressure. increasing the temperature of a gas increases the pressure and the energy of the gas particles. Boyle's. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. A calculate the force exerted by the book and then compute the area that is in contact with a surface. increasing the temperature of a gas increases the pressure and the energy of the gas particles. Boyle's law tells us that. pressure is caused. What Are Three Ways To Increase The Pressure Of A Gas.
From www.britannica.com
Properties and the theory of gas Britannica What Are Three Ways To Increase The Pressure Of A Gas A calculate the force exerted by the book and then compute the area that is in contact with a surface. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. Boyle's law tells us that. pressure is caused by the collisions between the atoms of gas and walls of the container as those. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Dalton’s Law The total pressure of a mixture of gases equals the What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. A calculate the force exerted by the book and then compute the area that is in contact with a surface. A decrease in container volume increases gas pressure. an. What Are Three Ways To Increase The Pressure Of A Gas.
From chemistryguru.com.sg
Ideal Gas Law and Applications What Are Three Ways To Increase The Pressure Of A Gas The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. A calculate the force exerted by the book and then compute the area that is in contact with a surface. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. An increase in temperature of a gas in. What Are Three Ways To Increase The Pressure Of A Gas.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID6918783 What Are Three Ways To Increase The Pressure Of A Gas A decrease in container volume increases gas pressure. An increase in temperature of a gas in a rigid container increases the pressure. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. Boyle's law tells us that. an increase in the number of gas molecules, while container volume stays constant, increases pressure. The. What Are Three Ways To Increase The Pressure Of A Gas.
From gcsephysicsninja.com
11. Heating gas at a constant pressure What Are Three Ways To Increase The Pressure Of A Gas A calculate the force exerted by the book and then compute the area that is in contact with a surface. Boyle's law tells us that. A decrease in container volume increases gas pressure. an increase in the number of gas molecules, while container volume stays constant, increases pressure. learn about and revise particle motion, gas pressure and the. What Are Three Ways To Increase The Pressure Of A Gas.
From learningschoolhanerydd2l.z21.web.core.windows.net
What Is Boyle's Law All About What Are Three Ways To Increase The Pressure Of A Gas A decrease in container volume increases gas pressure. an increase in the number of gas molecules, while container volume stays constant, increases pressure. Boyle's law tells us that. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. An increase in temperature of a gas in a rigid container increases the pressure. . What Are Three Ways To Increase The Pressure Of A Gas.
From www.britannica.com
Equation of state Definition, Ideal Gas, & Facts Britannica What Are Three Ways To Increase The Pressure Of A Gas increasing the temperature of a gas increases the pressure and the energy of the gas particles. an increase in the number of gas molecules, while container volume stays constant, increases pressure. Boyle's law tells us that. learn about and revise particle motion, gas pressure and the relationship between pressure and volume. A decrease in container volume increases. What Are Three Ways To Increase The Pressure Of A Gas.
From www.visionlearning.com
Properties of Gases Chemistry Visionlearning What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. A decrease in container volume increases gas pressure. increasing the temperature of a gas increases the pressure and the energy of the gas particles. A calculate the force exerted by the book and then compute the area that is in contact with a surface. Boyle's. What Are Three Ways To Increase The Pressure Of A Gas.
From www.savemyexams.co.uk
Gas Law Relationships (1.2.5) IB DP Chemistry SL Revision Notes 2016 What Are Three Ways To Increase The Pressure Of A Gas learn about and revise particle motion, gas pressure and the relationship between pressure and volume. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. An increase in temperature of a gas in a rigid container increases the pressure. A calculate the force exerted by the book and then. What Are Three Ways To Increase The Pressure Of A Gas.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry What Are Three Ways To Increase The Pressure Of A Gas An increase in temperature of a gas in a rigid container increases the pressure. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. A decrease in container volume increases gas pressure. an. What Are Three Ways To Increase The Pressure Of A Gas.
From www.youtube.com
Constant Pressure Heating of a gas YouTube What Are Three Ways To Increase The Pressure Of A Gas learn about and revise particle motion, gas pressure and the relationship between pressure and volume. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. increasing the temperature of a gas increases the pressure and the energy of the gas particles. Boyle's law tells us that. an. What Are Three Ways To Increase The Pressure Of A Gas.
From www.thoughtco.com
3 Ways To Increase the Pressure of a Gas What Are Three Ways To Increase The Pressure Of A Gas A calculate the force exerted by the book and then compute the area that is in contact with a surface. Boyle's law tells us that. pressure is caused by the collisions between the atoms of gas and walls of the container as those atoms. an increase in the number of gas molecules, while container volume stays constant, increases. What Are Three Ways To Increase The Pressure Of A Gas.