Lead Chloride Carbonate . It is not soluble in ammonia solution or dilute acid solutions. Learn more about lead precipitates,. Find the solubility of sodium chloride. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. The formula is pbcl2 (s). It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution.
from www.bartleby.com
Find the solubility of sodium chloride. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn more about lead precipitates,. The formula is pbcl2 (s). Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. It is not soluble in ammonia solution or dilute acid solutions.
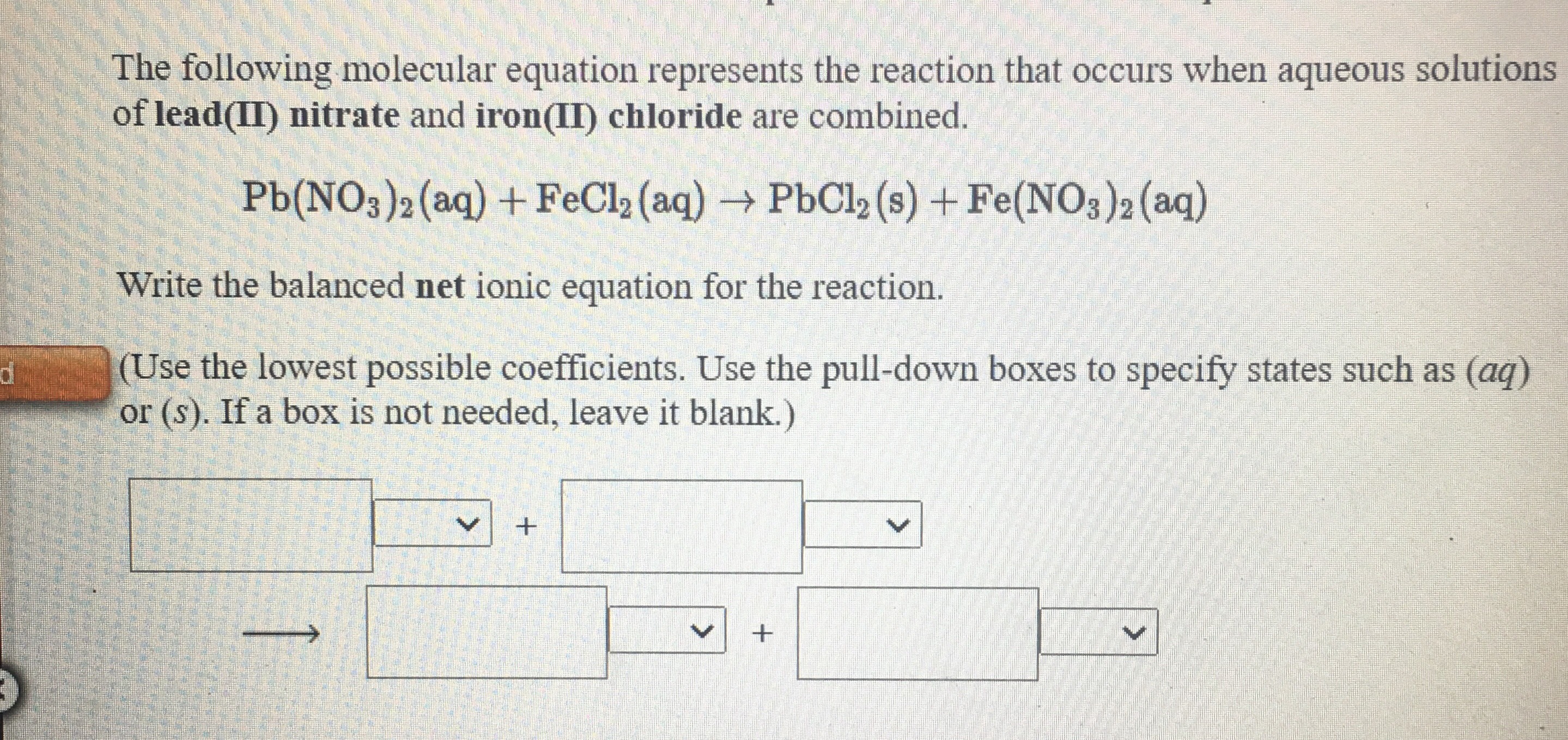
Answered When aqueous solutions of iron(II)… bartleby
Lead Chloride Carbonate Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. The formula is pbcl2 (s). Find the solubility of sodium chloride. Learn more about lead precipitates,. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It is not soluble in ammonia solution or dilute acid solutions. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution.
From www.indiamart.com
Lead Carbonate at best price in Thane by Nithyasri Chemicals ID Lead Chloride Carbonate Learn more about lead precipitates,. The formula is pbcl2 (s). It is not soluble in ammonia solution or dilute acid solutions. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Lead(ii) chloride precipitates from solution upon addition of. Lead Chloride Carbonate.
From www.indiamart.com
Lead Chloride powder, For Industrial, Packaging Type 50 Kg Hdpe Bag at Lead Chloride Carbonate Learn more about lead precipitates,. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It is not soluble in ammonia solution or dilute acid solutions. Find the solubility of sodium chloride. Lead chloride (pbcl2) is a white precipitate that is. Lead Chloride Carbonate.
From chemcraft.su
Lead(II) chloride, 99.5 pure p.a. chemcraft.su Lead Chloride Carbonate It is not soluble in ammonia solution or dilute acid solutions. The formula is pbcl2 (s). Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Learn how to make lead (ii) chloride as a white precipitate by adding. Lead Chloride Carbonate.
From www.youtube.com
How to Write the Net Ionic Equation for NaCl + Zn(NO3)2 = ZnCl2 + NaNO3 Lead Chloride Carbonate Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. The formula is pbcl2 (s). Learn more about lead precipitates,. It is not soluble in. Lead Chloride Carbonate.
From alchetron.com
Lead carbonate Alchetron, The Free Social Encyclopedia Lead Chloride Carbonate It is not soluble in ammonia solution or dilute acid solutions. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Find the solubility of sodium chloride. Learn how to calculate the solubility product and the. Lead Chloride Carbonate.
From www.alamy.com
Minerals Phosgenite (Lead Carbonate Chloride Stock Photo Alamy Lead Chloride Carbonate Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Learn more about lead precipitates,. The formula is pbcl2 (s). It is not soluble in ammonia solution or dilute acid solutions. Find. Lead Chloride Carbonate.
From testbook.com
Lead (II) Chloride Formula Structure, Properties, Uses & More Lead Chloride Carbonate Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Learn more about lead precipitates,. The formula is pbcl2 (s). Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate. Lead Chloride Carbonate.
From www.toppr.com
Lead II Chloride Formula Definition, Concepts and Examples Lead Chloride Carbonate Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. It is not soluble in ammonia solution or dilute acid solutions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate.. Lead Chloride Carbonate.
From collegedunia.com
Lead II Chloride Formula Properties, Structure, Examples Lead Chloride Carbonate It is not soluble in ammonia solution or dilute acid solutions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria.. Lead Chloride Carbonate.
From www.grainger.com
598630, F.W. 267.20, Lead Carbonate, Powder, Reagent, ACS 39G576 Lead Chloride Carbonate Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. The formula is pbcl2 (s). It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Find the solubility of sodium chloride.. Lead Chloride Carbonate.
From www.labdepotinc.com
Lead Carbonate, Powder, Reagent, ACS Lead Chloride Carbonate Learn more about lead precipitates,. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. It is not soluble in ammonia solution or dilute acid solutions. Lead(ii) chloride precipitates from solution upon addition of chloride. Lead Chloride Carbonate.
From www.youtube.com
How to Write the Formula for Lead (II) carbonate YouTube Lead Chloride Carbonate Learn more about lead precipitates,. Find the solubility of sodium chloride. It is not soluble in ammonia solution or dilute acid solutions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Lead chloride (pbcl2) is a white precipitate that is. Lead Chloride Carbonate.
From www.numerade.com
SOLVED Am asking on how a solid sample of lead ii chloride can be Lead Chloride Carbonate Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. It is not soluble in ammonia solution or dilute acid solutions. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl). Lead Chloride Carbonate.
From www.toppr.com
An aqueous solution of sodium salt (P) produce a white precipitate (Q Lead Chloride Carbonate Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Find the solubility of sodium chloride. Learn more about lead precipitates,. It is not soluble in ammonia solution or dilute acid solutions. Learn how. Lead Chloride Carbonate.
From www.ottokemi.com
Lead carbonate, basic 1319466 Manufacturers & Suppliers in India Lead Chloride Carbonate Find the solubility of sodium chloride. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. It is not soluble in ammonia solution or dilute acid solutions. Learn how to calculate the. Lead Chloride Carbonate.
From www.indiamart.com
Lead Carbonate at best price in Vadodara ID 26746766248 Lead Chloride Carbonate Learn more about lead precipitates,. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Find the. Lead Chloride Carbonate.
From www.ottokemi.com
Lead(II) carbonate Lead carbonate, GR 99+ 598630 Lead Lead Chloride Carbonate Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Learn more about lead precipitates,. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn. Lead Chloride Carbonate.
From www.slideserve.com
PPT Number One Sodium chloride solution + lead (II) acetate solutions Lead Chloride Carbonate Find the solubility of sodium chloride. It is not soluble in ammonia solution or dilute acid solutions. The formula is pbcl2 (s). Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. It describes the. Lead Chloride Carbonate.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Carbonate Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. It is not soluble in ammonia solution or dilute acid solutions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Learn how to make. Lead Chloride Carbonate.
From www.guidechem.com
CAS 1319466 Lead subcarbonate Manufacturers,suppliers,fob price Lead Chloride Carbonate Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Learn more about lead precipitates,. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Find the solubility of sodium chloride.. Lead Chloride Carbonate.
From www.labdepotinc.com
Barium Carbonate Purified Lead Chloride Carbonate Find the solubility of sodium chloride. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Learn more about lead precipitates,. It is not soluble in ammonia solution or dilute acid solutions. The formula is pbcl2 (s). It describes. Lead Chloride Carbonate.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Carbonate Learn more about lead precipitates,. The formula is pbcl2 (s). Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. It. Lead Chloride Carbonate.
From www.tessshebaylo.com
Write The Balanced Chemical Equation For Reaction Of Sodium Carbonate Lead Chloride Carbonate It is not soluble in ammonia solution or dilute acid solutions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali. Lead Chloride Carbonate.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Chloride Carbonate Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It is not soluble in ammonia solution or dilute acid solutions. The formula is pbcl2 (s). Learn how to calculate the solubility product and the solubility. Lead Chloride Carbonate.
From www.exportersindia.com
Lead Carbonate, Purity 99, Packaging Type HDPE Liner Bag at Rs 240 Lead Chloride Carbonate Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. The formula is pbcl2 (s). Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Lead chloride (pbcl2) is a white precipitate that. Lead Chloride Carbonate.
From www.numerade.com
SOLVED ILead(II) acetate and sodium carbonate (3Lead(II) acetate and Lead Chloride Carbonate Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Learn how to make lead (ii) chloride as a white precipitate. Lead Chloride Carbonate.
From www.anronchemicals.net
Lead Carbonate Manufacturer from Mumbai Lead Chloride Carbonate It is not soluble in ammonia solution or dilute acid solutions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. The formula. Lead Chloride Carbonate.
From www.grainger.com
598630, F.W. 267.20, Lead Carbonate, Powder, Reagent, ACS 39G578 Lead Chloride Carbonate The formula is pbcl2 (s). Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Learn more about lead precipitates,. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to. Lead Chloride Carbonate.
From www.dreamstime.com
PbCO3 Lead Carbonate CAS 598630 Chemical Substance in White Plastic Lead Chloride Carbonate Learn more about lead precipitates,. The formula is pbcl2 (s). Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. Find the solubility of sodium chloride. Learn how to calculate the solubility product and the solubility. Lead Chloride Carbonate.
From www.indiamart.com
Laboratory Lead Carbonate Liquid at Rs 120/bottle Lead Carbonate in Lead Chloride Carbonate It is not soluble in ammonia solution or dilute acid solutions. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Learn more about lead precipitates,. Lead(ii) chloride precipitates from solution upon addition of chloride. Lead Chloride Carbonate.
From www.numerade.com
SOLVED Double replacements Aluminum bromide and Iithium hydroxide Zinc Lead Chloride Carbonate Find the solubility of sodium chloride. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. It is not soluble in. Lead Chloride Carbonate.
From www.chegg.com
Solved Precipitation Reactions Results/Observations Lead Chloride Carbonate The formula is pbcl2 (s). Learn more about lead precipitates,. Find the solubility of sodium chloride. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Lead chloride (pbcl2) is a white precipitate that is. Lead Chloride Carbonate.
From www.numerade.com
SOLVED Write balanced complete chemical equation the complete ionic Lead Chloride Carbonate Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Find the solubility of sodium chloride. It is not soluble in ammonia solution or dilute acid solutions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Learn more about lead precipitates,. Lead chloride (pbcl2) is a white precipitate that is. Lead Chloride Carbonate.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Carbonate It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. The formula is pbcl2 (s). Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. It is not. Lead Chloride Carbonate.
From www.labdepotinc.com
Lead Carbonate, Basic Lead Chloride Carbonate Learn how to make lead (ii) chloride as a white precipitate by adding hydrochloric acid to lead (ii) nitrate solution. Lead chloride (pbcl2) is a white precipitate that is soluble in hot water and alkali hydroxides. The formula is pbcl2 (s). Learn how to calculate the solubility product and the solubility of salts using chemical equilibria. Lead(ii) chloride precipitates from. Lead Chloride Carbonate.