Beer's Law Lab Questions . “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Determine order of reaction by measuring the concentration of a reactant as a function of. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.
from www.chegg.com
Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring the concentration of a reactant as a function of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law).
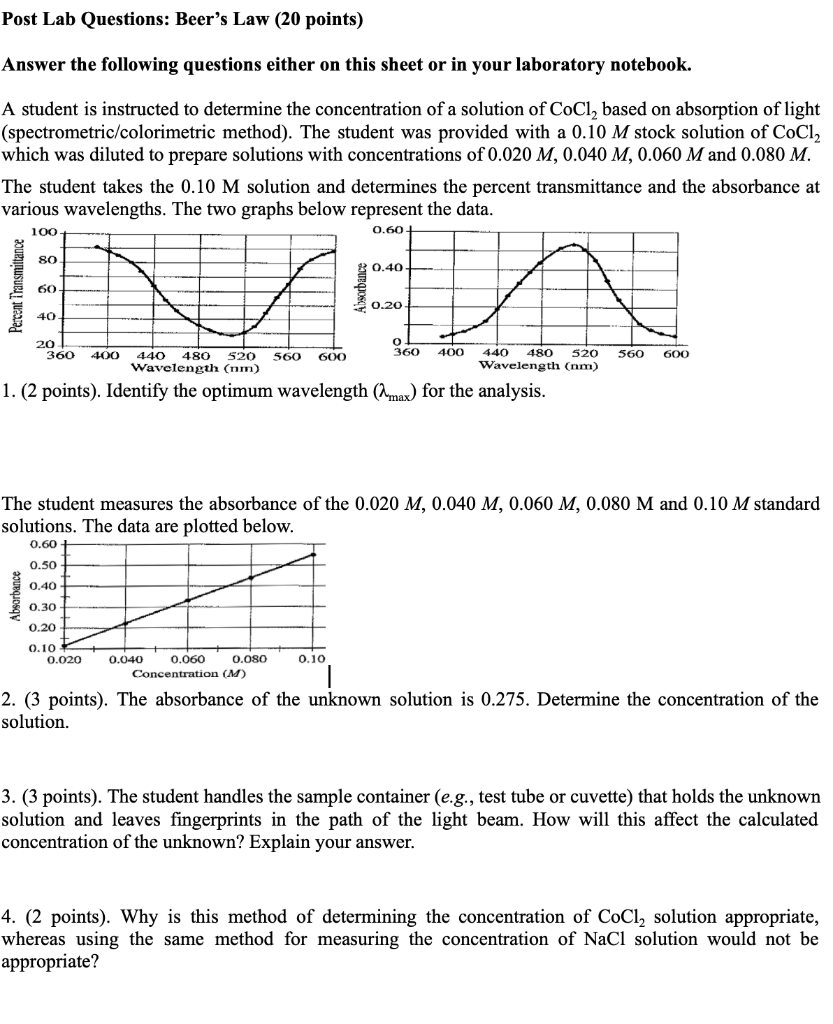
Solved Post Lab Questions Beer's Law (20 points) Answer the
Beer's Law Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring the concentration of a reactant as a function of. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.
From www.studypool.com
SOLUTION 2 beer lambert law complete Studypool Beer's Law Lab Questions Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Determine order of reaction by measuring the concentration of a reactant as a function of. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Study with quizlet. Beer's Law Lab Questions.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Lab Questions Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the. Beer's Law Lab Questions.
From www.chegg.com
Solved Name Experiment 6 Beer's Law Pre Lab Questions 1. Beer's Law Lab Questions Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated. Beer's Law Lab Questions.
From www.chegg.com
Solved Beer's Law Part 2 Concentration from Color Data & Beer's Law Lab Questions Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Study. Beer's Law Lab Questions.
From studylib.net
Beer's Law Lab Beer's Law Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Study with. Beer's Law Lab Questions.
From www.chegg.com
Solved Lab Report Beer's Law Part 1 Determination of the Beer's Law Lab Questions Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for. Beer's Law Lab Questions.
From www.chegg.com
Open the Beer's Law Lab simulation on your laptop or Beer's Law Lab Questions Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the less the light that passes through.” make. Beer's Law Lab Questions.
From www.chegg.com
Solved Beer's Law Name Date PRELAB QUESTIONS 1. What is the Beer's Law Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated. Beer's Law Lab Questions.
From www.chegg.com
Solved Post Lab Questions Beer's Law (20 points) Answer the Beer's Law Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Determine order of reaction by measuring the concentration of a reactant as a function of. Consider monochromatic light of a given intensity incident on a. Beer's Law Lab Questions.
From www.studocu.com
Beer’s Law PreLab Worksheet Complete the worksheet and submit it to Beer's Law Lab Questions Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Determine order of reaction by measuring the concentration of a reactant as a function of. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Calculate the gradient (m) for the equation of the line of the. Beer's Law Lab Questions.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Lab Questions Determine order of reaction by measuring the concentration of a reactant as a function of. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. The amount of light that a species absorbs in a spectroscopic transition. Beer's Law Lab Questions.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Lab Questions Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Explore beer's law by creating colorful solutions and measuring light absorption. Beer's Law Lab Questions.
From www.chegg.com
Solved Beer's Law Lab Leaming Objective 1) Develop a Beer's Law Lab Questions Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring the concentration of a reactant as a function of. Explore beer's law by creating colorful solutions and measuring. Beer's Law Lab Questions.
From studylib.net
Beer's Law Lab Beer's Law Lab Questions Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a. Beer's Law Lab Questions.
From www.chegg.com
Solved Lab Partner Instructor Experiment 10 Beer's Law Beer's Law Lab Questions Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Determine order of reaction by measuring the concentration of. Beer's Law Lab Questions.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Beer's Law Lab Questions Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Determine order of reaction by measuring the concentration of a reactant as a function of. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The amount of light that a. Beer's Law Lab Questions.
From study.com
Quiz & Worksheet Spectrophotometry & Beer's Law Beer's Law Lab Questions Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Determine order of reaction by. Beer's Law Lab Questions.
From studylib.net
Lab 7 Beer's Law Beer's Law Lab Questions Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Determine order of reaction by measuring the concentration of a reactant as a function of. Calculate the gradient (m) for the equation. Beer's Law Lab Questions.
From www.chegg.com
A 3 paged lab over the Beer's Law screen. Its a quick Beer's Law Lab Questions “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Determine order of reaction by measuring the concentration of a reactant as a function of. Study with quizlet. Beer's Law Lab Questions.
From www.chegg.com
Solved Describe what each term in the Beer's Law equation Beer's Law Lab Questions Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Study. Beer's Law Lab Questions.
From www.chegg.com
Solved Post Lab Questions Beer's Law (20 points) Answer the Beer's Law Lab Questions Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Determine order of reaction by measuring the concentration of a reactant as a function of. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Consider monochromatic light. Beer's Law Lab Questions.
From www.chegg.com
Solved Prelab LO7 Beer's Law (10pts) Name Please review the Beer's Law Lab Questions Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Explore beer's. Beer's Law Lab Questions.
From www.scribd.com
Beer's Law Lab Questions PDF Absorbance Spectrophotometry Beer's Law Lab Questions Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Determine order of reaction by measuring the concentration of a reactant as a function of. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The amount of light that a species absorbs in a spectroscopic transition can be related. Beer's Law Lab Questions.
From www.chegg.com
Solved Experiment 12 Beer's Law PreLab Report Sheet Name Beer's Law Lab Questions Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Determine order of reaction by measuring the concentration of a reactant. Beer's Law Lab Questions.
From www.chegg.com
Solved PreLab Assignment Beer's Law This experiment covers Beer's Law Lab Questions Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the. Beer's Law Lab Questions.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Lab Questions “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Consider. Beer's Law Lab Questions.
From studylib.net
Beer's Law Lab Beer's Law Lab Questions Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest. Beer's Law Lab Questions.
From www.chegg.com
Solved Lab 5 2162 Beer's Law and Standard Curves Prepared by Beer's Law Lab Questions Determine order of reaction by measuring the concentration of a reactant as a function of. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Explore beer's law by creating colorful solutions and measuring. Beer's Law Lab Questions.
From www.chegg.com
Solved Beer's Law Lab You must show your work. No work = no Beer's Law Lab Questions Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Determine order of reaction by measuring the concentration of a reactant as a function of. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The amount of light that a. Beer's Law Lab Questions.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Beer's Law Lab Questions Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Calculate the gradient (m) for. Beer's Law Lab Questions.
From www.chegg.com
Solved Spectrophotometry and Beer's Law spremenities Lab Beer's Law Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds,. Beer's Law Lab Questions.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Beer's Law Lab Questions Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Calculate the. Beer's Law Lab Questions.
From www.chegg.com
Solved BEERS LAWTransmi COLORIMETRYBEER'S LAW LAB REPORT Beer's Law Lab Questions Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Determine order of reaction by measuring the concentration of a reactant as a function of. Study with quizlet and memorize flashcards containing terms like purpose of experiment:, pi and sigma bonds, color of unknown solution:. The amount of. Beer's Law Lab Questions.
From www.chegg.com
Solved Beer's Law Name Date PRELAB QUESTIONS 1. What is the Beer's Law Lab Questions Determine order of reaction by measuring the concentration of a reactant as a function of. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Explore beer's law by creating colorful solutions and measuring light absorption and. Beer's Law Lab Questions.
From www.chegg.com
Solved Beer's Law Name Date PRELAB QUESTIONS 1. What is the Beer's Law Lab Questions Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Study with quizlet and memorize. Beer's Law Lab Questions.