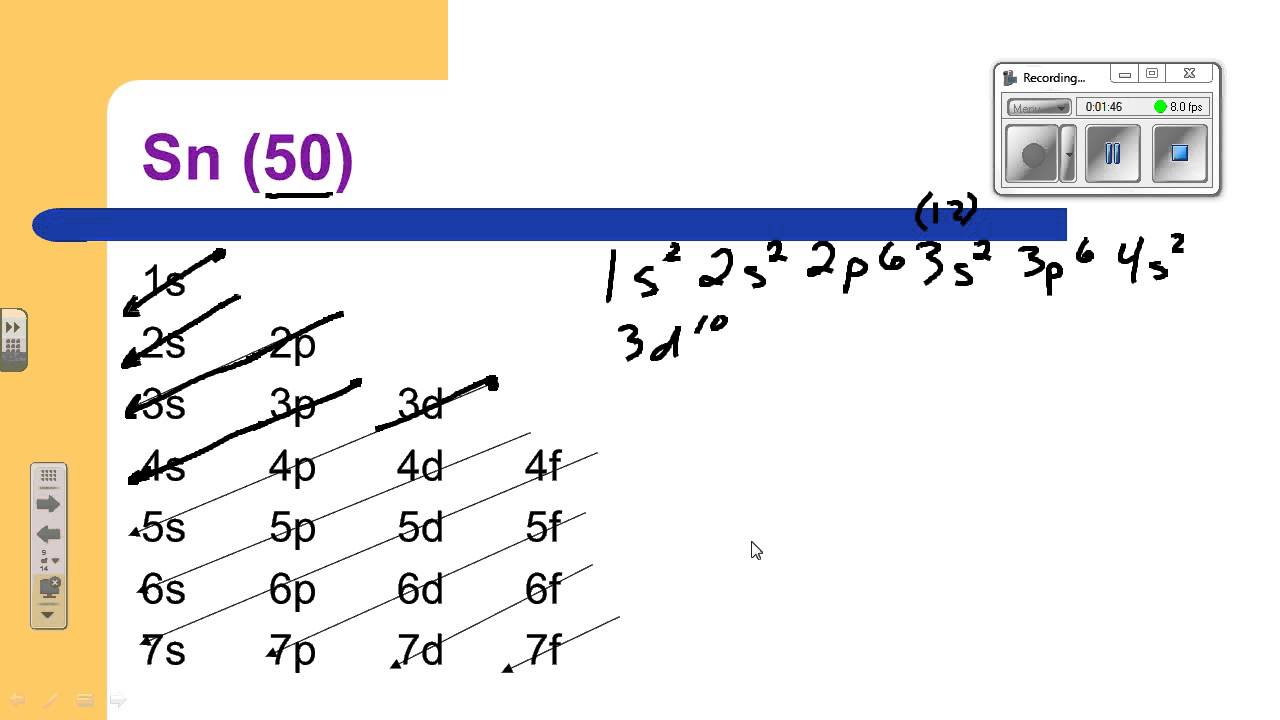
Tin Outer Electron Configuration . Elements are organised into blocks by the orbital type in which the outer electrons are found. Before drawing the orbital diagram, you should know the three general. Indium ← tin → antimony These blocks are named for the characteristic spectra. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. Full electron configuration of tin: Calculate the maximum number of electrons each subshell can hold using the formula: The shorthand electron configuration (or noble gas configuration) as well as. This electron arrangement and electron configuration indicates that the. Now in the next step, start drawing the orbital diagram for tin. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Where, ℓ = azimuthal quantum number of the subshell. Electron configuration chart of all elements is mentioned in the table below.
from periodictable.me
Indium ← tin → antimony Now in the next step, start drawing the orbital diagram for tin. Full electron configuration of tin: These blocks are named for the characteristic spectra. Where, ℓ = azimuthal quantum number of the subshell. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Before drawing the orbital diagram, you should know the three general. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. Electron configuration chart of all elements is mentioned in the table below. Elements are organised into blocks by the orbital type in which the outer electrons are found.
Tin Electron Configuration (Sn) with Orbital Diagram
Tin Outer Electron Configuration Before drawing the orbital diagram, you should know the three general. This electron arrangement and electron configuration indicates that the. Where, ℓ = azimuthal quantum number of the subshell. Indium ← tin → antimony Now in the next step, start drawing the orbital diagram for tin. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Before drawing the orbital diagram, you should know the three general. Calculate the maximum number of electrons each subshell can hold using the formula: The shorthand electron configuration (or noble gas configuration) as well as. Full electron configuration of tin: Elements are organised into blocks by the orbital type in which the outer electrons are found. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. These blocks are named for the characteristic spectra. Electron configuration chart of all elements is mentioned in the table below.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Tin Outer Electron Configuration These blocks are named for the characteristic spectra. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Calculate the maximum number of electrons each subshell can hold using the formula: Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic.. Tin Outer Electron Configuration.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Outer Electron Configuration Electron configuration chart of all elements is mentioned in the table below. Elements are organised into blocks by the orbital type in which the outer electrons are found. Calculate the maximum number of electrons each subshell can hold using the formula: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s. Tin Outer Electron Configuration.
From www.inspiritvr.com
Electron Configuration Study Guide Inspirit Tin Outer Electron Configuration This electron arrangement and electron configuration indicates that the. Electron configuration chart of all elements is mentioned in the table below. Where, ℓ = azimuthal quantum number of the subshell. These blocks are named for the characteristic spectra. Indium ← tin → antimony Tin is a chemical element with atomic number 50 which means there are 50 protons and 50. Tin Outer Electron Configuration.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Outer Electron Configuration Full electron configuration of tin: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Indium ← tin → antimony And the electron configuration of tin is. Tin Outer Electron Configuration.
From www.alamy.com
Sn Tin, Periodic Table of the Elements, Shell Structure of Tin Tin Outer Electron Configuration Where, ℓ = azimuthal quantum number of the subshell. These blocks are named for the characteristic spectra. The shorthand electron configuration (or noble gas configuration) as well as. Elements are organised into blocks by the orbital type in which the outer electrons are found. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10. Tin Outer Electron Configuration.
From www.chegg.com
Solved Identify the general outer electron configuration for Tin Outer Electron Configuration And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Indium ← tin → antimony Electron configuration chart of all elements is mentioned in the table below. Now in the next. Tin Outer Electron Configuration.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Outer Electron Configuration These blocks are named for the characteristic spectra. Where, ℓ = azimuthal quantum number of the subshell. Indium ← tin → antimony And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Now in the next step, start drawing the orbital diagram for tin. 1s 2 2s 2 2p 6 3s 2. Tin Outer Electron Configuration.
From brightpathtutors.org
Electron Configuration Basic introduction En doğru 1s 2s 2p ile Tin Outer Electron Configuration These blocks are named for the characteristic spectra. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Elements are organised into blocks by the orbital type in which the outer electrons are found. Electron configuration chart of all elements is mentioned in the table below. Now in the next step, start. Tin Outer Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Tin Outer Electron Configuration Now in the next step, start drawing the orbital diagram for tin. These blocks are named for the characteristic spectra. The shorthand electron configuration (or noble gas configuration) as well as. This electron arrangement and electron configuration indicates that the. Where, ℓ = azimuthal quantum number of the subshell. Elements are organised into blocks by the orbital type in which. Tin Outer Electron Configuration.
From www.youtube.com
Sn^2+,Sn^4+ and Sn (Tin and Tin Ions) Electron ConfigurationCrash Tin Outer Electron Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. Full electron configuration of tin: These blocks are named for the characteristic spectra. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Calculate the maximum number. Tin Outer Electron Configuration.
From www.youtube.com
How to Find the Valence Electrons for Tin (Sn) YouTube Tin Outer Electron Configuration Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Now in the next step, start drawing the orbital diagram for tin. Full electron configuration of tin: Elements are organised into blocks by the orbital type in which the outer electrons are found. Where, ℓ = azimuthal quantum number. Tin Outer Electron Configuration.
From www.webelements.com
Elements Periodic Table » Tin » properties of free atoms Tin Outer Electron Configuration Full electron configuration of tin: These blocks are named for the characteristic spectra. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Electron configuration chart of all elements is mentioned in the table below. Before drawing the orbital diagram, you should know the three general. Now in the. Tin Outer Electron Configuration.
From www.slideserve.com
PPT Unit 6 Chapters 1112. Pages 295366 ATOMIC ELECTRON Tin Outer Electron Configuration Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Electron configuration chart of all elements is mentioned in the table below. This electron arrangement and electron configuration indicates that the. Before drawing the orbital diagram, you should know the three general. And the electron configuration of tin is. Tin Outer Electron Configuration.
From www.youtube.com
Electron Configuration of Tin Sn Lesson YouTube Tin Outer Electron Configuration Electron configuration chart of all elements is mentioned in the table below. Elements are organised into blocks by the orbital type in which the outer electrons are found. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Now in the next step, start drawing the orbital diagram for. Tin Outer Electron Configuration.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Tin Outer Electron Configuration Full electron configuration of tin: Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Calculate the maximum number of electrons each subshell can hold using the formula: This electron arrangement and electron configuration indicates that the. Before drawing the orbital diagram, you should know the three. Tin Outer Electron Configuration.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Outer Electron Configuration Indium ← tin → antimony Where, ℓ = azimuthal quantum number of the subshell. The shorthand electron configuration (or noble gas configuration) as well as. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. This electron arrangement and electron configuration indicates that the. Calculate the maximum number of electrons each subshell. Tin Outer Electron Configuration.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost Tin Outer Electron Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. These blocks are named for the characteristic spectra. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. And the electron configuration of tin is 1s2 2s2. Tin Outer Electron Configuration.
From sciencenotes.org
List of Electron Configurations of Elements Tin Outer Electron Configuration Where, ℓ = azimuthal quantum number of the subshell. Full electron configuration of tin: Electron configuration chart of all elements is mentioned in the table below. This electron arrangement and electron configuration indicates that the. Before drawing the orbital diagram, you should know the three general. Tin is a chemical element with atomic number 50 which means there are 50. Tin Outer Electron Configuration.
From valenceelectrons.com
Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+) Tin Outer Electron Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. The shorthand electron configuration (or noble gas configuration) as well as. This electron arrangement and electron configuration indicates that the. Where, ℓ = azimuthal quantum number of the subshell. Calculate the maximum number of electrons each subshell can. Tin Outer Electron Configuration.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Tin Outer Electron Configuration Now in the next step, start drawing the orbital diagram for tin. This electron arrangement and electron configuration indicates that the. Calculate the maximum number of electrons each subshell can hold using the formula: Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. The shorthand electron configuration (or. Tin Outer Electron Configuration.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration Tin Outer Electron Configuration Now in the next step, start drawing the orbital diagram for tin. Indium ← tin → antimony These blocks are named for the characteristic spectra. This electron arrangement and electron configuration indicates that the. Where, ℓ = azimuthal quantum number of the subshell. Elements are organised into blocks by the orbital type in which the outer electrons are found. And. Tin Outer Electron Configuration.
From www.newtondesk.com
Tin Sn (Element 50) of Periodic Table Periodic Table FlashCards Tin Outer Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Elements are organised into blocks by the orbital type in which the outer electrons are found. Indium ← tin → antimony Now in the next step, start drawing the orbital diagram for tin. And the electron configuration. Tin Outer Electron Configuration.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Tin Outer Electron Configuration Electron configuration chart of all elements is mentioned in the table below. Elements are organised into blocks by the orbital type in which the outer electrons are found. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. This electron arrangement and electron configuration indicates that the. Full. Tin Outer Electron Configuration.
From www.sciencephoto.com
Tin, atomic structure Stock Image C018/3731 Science Photo Library Tin Outer Electron Configuration Electron configuration chart of all elements is mentioned in the table below. Full electron configuration of tin: Indium ← tin → antimony And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. These blocks are named for the characteristic spectra. Calculate the maximum number of electrons each subshell can hold using the. Tin Outer Electron Configuration.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Outer Electron Configuration Calculate the maximum number of electrons each subshell can hold using the formula: Full electron configuration of tin: Before drawing the orbital diagram, you should know the three general. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. 1s 2 2s 2 2p 6 3s 2 3p 6. Tin Outer Electron Configuration.
From wiringlistvoltairean.z14.web.core.windows.net
How To Write Electron Configuration Diagrams Tin Outer Electron Configuration And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. Full electron configuration of tin: Where, ℓ = azimuthal quantum number of the subshell. This electron arrangement and electron configuration. Tin Outer Electron Configuration.
From chemistrytalk.org
Electron Shells ChemTalk Tin Outer Electron Configuration Elements are organised into blocks by the orbital type in which the outer electrons are found. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Indium ← tin → antimony Calculate the maximum number of electrons each subshell can hold using the formula: Where, ℓ = azimuthal quantum number of the. Tin Outer Electron Configuration.
From warreninstitute.org
Outer Electron Box Diagram For A Cation Simplify And Visualize Tin Outer Electron Configuration These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Now in the next step, start drawing the orbital diagram for tin. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Calculate the maximum number of electrons. Tin Outer Electron Configuration.
From www.vectorstock.com
Symbol and electron diagram for tin Royalty Free Vector Tin Outer Electron Configuration Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra. Electron configuration chart of all elements is mentioned in the table below. Calculate the maximum. Tin Outer Electron Configuration.
From www.numerade.com
SOLVEDList the outer electron configuration for each column in the Tin Outer Electron Configuration Indium ← tin → antimony These blocks are named for the characteristic spectra. Where, ℓ = azimuthal quantum number of the subshell. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. Before drawing the orbital diagram, you should know the three general. And the electron configuration of. Tin Outer Electron Configuration.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Outer Electron Configuration Calculate the maximum number of electrons each subshell can hold using the formula: Where, ℓ = azimuthal quantum number of the subshell. The shorthand electron configuration (or noble gas configuration) as well as. Full electron configuration of tin: And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. This electron arrangement and. Tin Outer Electron Configuration.
From www.thoughtco.com
Electron Configuration Chart Tin Outer Electron Configuration And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Electron configuration chart of all elements is mentioned in the table below. Now in the next step, start drawing the orbital diagram for tin. This electron arrangement and electron configuration indicates that the. 1s 2 2s 2 2p 6 3s 2 3p. Tin Outer Electron Configuration.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Outer Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Where, ℓ = azimuthal quantum number of the subshell. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2.. Tin Outer Electron Configuration.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Tin Outer Electron Configuration Elements are organised into blocks by the orbital type in which the outer electrons are found. Full electron configuration of tin: Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2.. Tin Outer Electron Configuration.
From valenceelectrons.com
Complete Electron Configuration for Xenon (Xe) Tin Outer Electron Configuration And the electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. These blocks are named for the characteristic spectra. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. Elements are organised into blocks by the orbital type in which the. Tin Outer Electron Configuration.