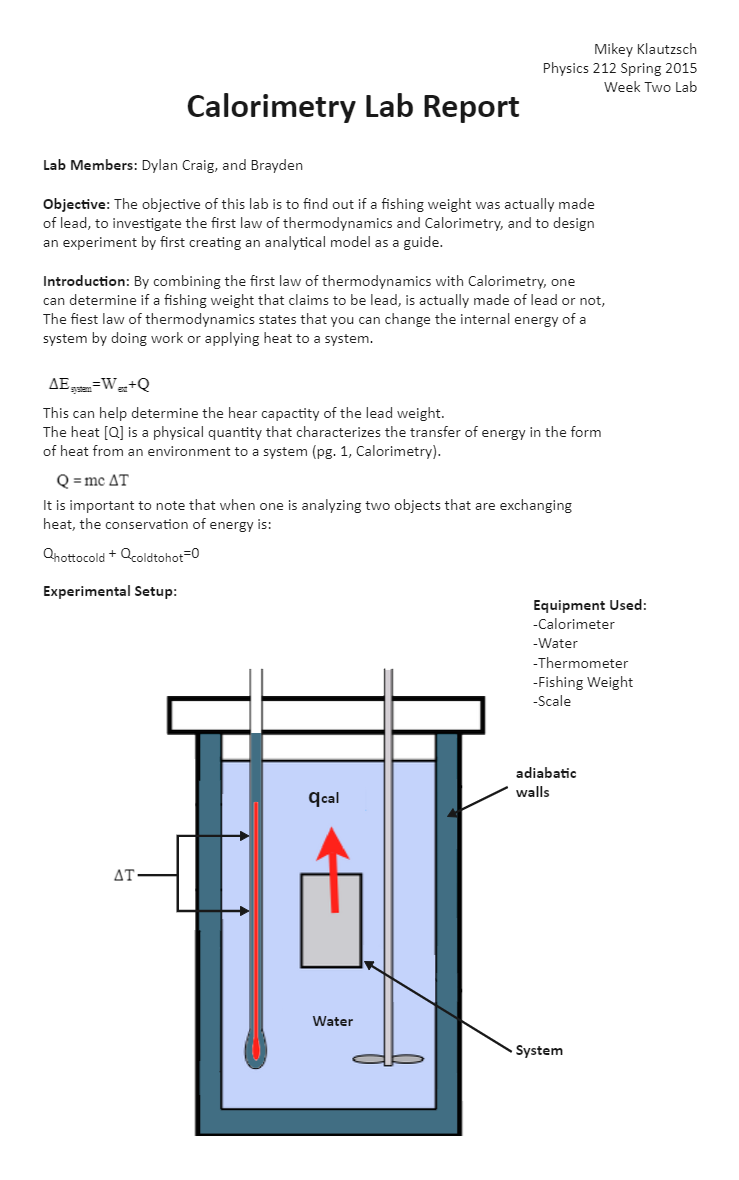
Calorimeter Chemistry Lab . you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. calorimetry is the science of measuring heat flow. Values in a hess’s law calculation to. Heat is defined as thermal energy flowing from an object at a higher. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. in the laboratory, heat flow is measured in an apparatus called a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. A calorimeter is a device. Values of two reactions using the technique of constant pressure calorimetry.
from www.edrawmax.com
A calorimeter is a device. in the laboratory, heat flow is measured in an apparatus called a calorimeter. calorimetry is the science of measuring heat flow. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Values of two reactions using the technique of constant pressure calorimetry. Heat is defined as thermal energy flowing from an object at a higher. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Values in a hess’s law calculation to.
Calorimetry Lab Report EdrawMax Template
Calorimeter Chemistry Lab A calorimeter is a device. in the laboratory, heat flow is measured in an apparatus called a calorimeter. Heat is defined as thermal energy flowing from an object at a higher. A calorimeter is a device. Values of two reactions using the technique of constant pressure calorimetry. you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Values in a hess’s law calculation to. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the science of measuring heat flow.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Chemistry Lab you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. calorimetry is the science of measuring heat flow. Values in. Calorimeter Chemistry Lab.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimeter Chemistry Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Heat is defined as thermal energy flowing from an object at a higher. you can determine energy content by. Calorimeter Chemistry Lab.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter Chemistry Lab Heat is defined as thermal energy flowing from an object at a higher. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the science of measuring heat flow. Values of two reactions using the technique of constant pressure calorimetry. calorimetry is the study of heat. Calorimeter Chemistry Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimeter Chemistry Lab Values of two reactions using the technique of constant pressure calorimetry. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a.. Calorimeter Chemistry Lab.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimeter Chemistry Lab you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a. calorimetry is the science of measuring heat flow. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic. Calorimeter Chemistry Lab.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Chemistry Lab Values in a hess’s law calculation to. A calorimeter is a device. in the laboratory, heat flow is measured in an apparatus called a calorimeter. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. a calorimeter is a device used. Calorimeter Chemistry Lab.
From saylordotorg.github.io
Calorimetry Calorimeter Chemistry Lab Values in a hess’s law calculation to. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. you can determine energy content by burning a known. Calorimeter Chemistry Lab.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter Chemistry Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Values of two reactions using the technique of constant pressure calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. you. Calorimeter Chemistry Lab.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimeter Chemistry Lab calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. you can determine energy content by burning a known mass of food and capturing the heat. Calorimeter Chemistry Lab.
From socratic.org
A student conducting a calorimetry investigation determines a negative Calorimeter Chemistry Lab in the laboratory, heat flow is measured in an apparatus called a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a.. Calorimeter Chemistry Lab.
From www.coleparmer.com
IKA C2000 Calorimeter Basic V1 230V 440 x 500 x 450 mm) from ColeParmer Calorimeter Chemistry Lab Heat is defined as thermal energy flowing from an object at a higher. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the science of measuring heat flow. A calorimeter is a device. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimeter Chemistry Lab.
From www.youtube.com
Calorimetry Lab1 YouTube Calorimeter Chemistry Lab calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Values in a hess’s law calculation to. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the. Calorimeter Chemistry Lab.
From cider.uoregon.edu
Calorimetry Computer Simulation HTML5 CIDER Calorimeter Chemistry Lab in the laboratory, heat flow is measured in an apparatus called a calorimeter. Heat is defined as thermal energy flowing from an object at a higher. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. calorimetry is the science of. Calorimeter Chemistry Lab.
From kaffee.50webs.com
Lab Calorimetry Calorimeter Chemistry Lab Values of two reactions using the technique of constant pressure calorimetry. Heat is defined as thermal energy flowing from an object at a higher. calorimetry is the science of measuring heat flow. A calorimeter is a device. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this. Calorimeter Chemistry Lab.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Calorimeter Chemistry Lab Heat is defined as thermal energy flowing from an object at a higher. in the laboratory, heat flow is measured in an apparatus called a calorimeter. Values in a hess’s law calculation to. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its. Calorimeter Chemistry Lab.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Chemistry Lab calorimetry is the science of measuring heat flow. Values in a hess’s law calculation to. A calorimeter is a device. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Values of two reactions using the technique of constant pressure calorimetry. in the laboratory, heat flow is measured. Calorimeter Chemistry Lab.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimeter Chemistry Lab Values of two reactions using the technique of constant pressure calorimetry. Values in a hess’s law calculation to. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat is defined as thermal energy flowing from an object at a higher. calorimetry is the science of measuring heat flow.. Calorimeter Chemistry Lab.
From exoxbgvei.blob.core.windows.net
Calorimeter Laboratory at Clara Carter blog Calorimeter Chemistry Lab calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. calorimetry is the science of measuring heat flow. in the laboratory, heat flow is measured in an apparatus called a calorimeter. Values of two reactions using the technique of constant pressure calorimetry. calorimetry is. Calorimeter Chemistry Lab.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimeter Chemistry Lab Values in a hess’s law calculation to. calorimetry is the science of measuring heat flow. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. Calorimeter Chemistry Lab.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimeter Chemistry Lab For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. Values in a hess’s law calculation to. Values of two reactions using the technique of constant pressure calorimetry. you can determine energy content by burning a known mass of food and capturing. Calorimeter Chemistry Lab.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Chemistry Lab A calorimeter is a device. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Values in a hess’s law calculation to. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. Heat. Calorimeter Chemistry Lab.
From www.youtube.com
Calorimetry Experiment YouTube Calorimeter Chemistry Lab Heat is defined as thermal energy flowing from an object at a higher. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to. Calorimeter Chemistry Lab.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Calorimeter Chemistry Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. Values of two reactions using the technique of constant pressure calorimetry. in. Calorimeter Chemistry Lab.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimeter Chemistry Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in the laboratory, heat flow is measured in an apparatus called a calorimeter. you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a.. Calorimeter Chemistry Lab.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Chemistry Lab in the laboratory, heat flow is measured in an apparatus called a calorimeter. you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a. Values of two reactions using the technique of constant pressure calorimetry. calorimetry is the science of measuring heat flow.. Calorimeter Chemistry Lab.
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Calorimeter Chemistry Lab calorimetry is the science of measuring heat flow. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. A calorimeter is a device. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. Calorimeter Chemistry Lab.
From www.eiscolabs.com
Calorimeter, Aluminium — Eisco Labs Calorimeter Chemistry Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat is defined as thermal energy flowing from an object at a higher. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature.. Calorimeter Chemistry Lab.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Calorimeter Chemistry Lab calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. in the laboratory, heat flow is measured in an apparatus called a calorimeter. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. calorimetry is. Calorimeter Chemistry Lab.
From exoxbgvei.blob.core.windows.net
Calorimeter Laboratory at Clara Carter blog Calorimeter Chemistry Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in the laboratory, heat flow is measured in an apparatus called a calorimeter. you can determine energy content by burning a known mass of food and capturing the heat released to a known mass of water in a.. Calorimeter Chemistry Lab.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Concept Calorimeter Chemistry Lab A calorimeter is a device. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Heat is defined as thermal energy flowing from an object at a higher. in the laboratory, heat flow is measured in an apparatus called a calorimeter. Values of two reactions using the technique of. Calorimeter Chemistry Lab.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimeter Chemistry Lab calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Chemistry Lab.
From www.laboratory-equipment.com
Laboratory Calorimeters from IKA Calorimeter Chemistry Lab in the laboratory, heat flow is measured in an apparatus called a calorimeter. Heat is defined as thermal energy flowing from an object at a higher. calorimetry is the science of measuring heat flow. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the. Calorimeter Chemistry Lab.
From users.highland.edu
Calorimetry Calorimeter Chemistry Lab Heat is defined as thermal energy flowing from an object at a higher. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device. For example,. Calorimeter Chemistry Lab.
From www.excedr.com
What Is a Calorimeter & How Is It Used in a Lab? Calorimeter Chemistry Lab For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. Values in a hess’s law calculation to. A calorimeter is a device. calorimetry is the science of measuring heat flow. calorimetry is the study of heat transferred in a chemical reaction,. Calorimeter Chemistry Lab.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific Calorimeter Chemistry Lab calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Values of two reactions using the technique of constant pressure calorimetry. Heat is defined as thermal energy flowing from an object at a higher. A calorimeter is a device. calorimetry is the study of heat transferred in a chemical. Calorimeter Chemistry Lab.