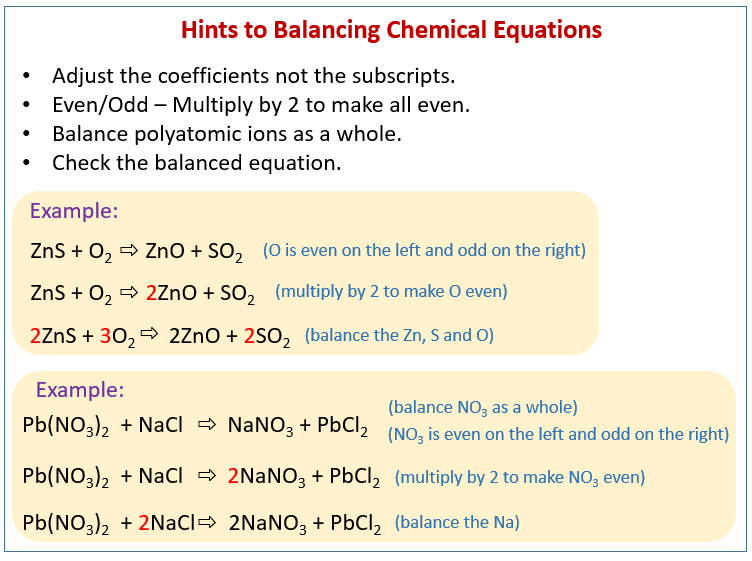
Explain Balancing Equations . the key to balancing chemical equations. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. Check that all the formulae. Balance a chemical equation when given the unbalanced. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. To balance equations on your own, follow these simple rules: explain the roles of subscripts and coefficients in chemical equations.
from www.onlinemathlearning.com
a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. To balance equations on your own, follow these simple rules: Balance a chemical equation when given the unbalanced. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. Check that all the formulae. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. the key to balancing chemical equations. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. explain the roles of subscripts and coefficients in chemical equations.
Balance Chemical Equations (solutions, examples, videos)
Explain Balancing Equations a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. the key to balancing chemical equations. explain the roles of subscripts and coefficients in chemical equations. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. Balance a chemical equation when given the unbalanced. Check that all the formulae. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. To balance equations on your own, follow these simple rules:
From www.youtube.com
How To Balance Chemical Equations YouTube Explain Balancing Equations To balance equations on your own, follow these simple rules: a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. explain the roles of subscripts and coefficients in chemical equations. a balanced equation is an equation for a chemical reaction in which the. Explain Balancing Equations.
From the-world-is-my-classroom.weebly.com
Unit 6.2 Solving Equations Using Balance Strategies MR. MARTÍNEZ'S Explain Balancing Equations the key to balancing chemical equations. Check that all the formulae. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. Balance a chemical equation when given the unbalanced. a. Explain Balancing Equations.
From www.sliderbase.com
Balancing Chemical Equations Presentation Chemistry Explain Balancing Equations a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. Balance a chemical equation when given the unbalanced. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. Check. Explain Balancing Equations.
From www.tes.com
Balancing equations explained Teaching Resources Explain Balancing Equations Check that all the formulae. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. Balance a chemical equation when given the unbalanced. To balance equations on your own, follow these simple rules: the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free Explain Balancing Equations The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. Balance a chemical equation when given the unbalanced. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. To balance equations on your own, follow these simple rules: Check that all the. Explain Balancing Equations.
From vhmsscience8.weebly.com
Balancing Chemical Equations VISTA HEIGHTS 8TH GRADE SCIENCE Explain Balancing Equations the key to balancing chemical equations. a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. the simplest and most generally. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Equations PowerPoint Presentation, free download ID Explain Balancing Equations To balance equations on your own, follow these simple rules: a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is. Explain Balancing Equations.
From www.youtube.com
Balancing Chemical Equations UPDATED Chemistry Tutorial YouTube Explain Balancing Equations the key to balancing chemical equations. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. a balanced equation is a chemical equation in. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Equations PowerPoint Presentation, free download ID Explain Balancing Equations a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. explain the roles of subscripts and coefficients in. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Equations PowerPoint Presentation, free download ID Explain Balancing Equations Balance a chemical equation when given the unbalanced. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. To balance equations on your own, follow. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Equations PowerPoint Presentation, free download ID Explain Balancing Equations Balance a chemical equation when given the unbalanced. Check that all the formulae. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. a balanced equation is an equation for a. Explain Balancing Equations.
From templatelab.com
49 Balancing Chemical Equations Worksheets [with Answers] Explain Balancing Equations the key to balancing chemical equations. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. explain the roles of subscripts and coefficients in chemical equations. a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. . Explain Balancing Equations.
From vhmsscience.weebly.com
Chemical Equations & Conservation VISTA HEIGHTS 8TH GRADE SCIENCE Explain Balancing Equations The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. explain the roles of subscripts and coefficients in chemical equations. the simplest and. Explain Balancing Equations.
From middleschoolscience.com
Balancing Equations A Hands on Activity Middle School Science Blog Explain Balancing Equations explain the roles of subscripts and coefficients in chemical equations. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. To balance equations on your own, follow these simple rules: a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the. Explain Balancing Equations.
From templatelab.com
49 Balancing Chemical Equations Worksheets [with Answers] Explain Balancing Equations Check that all the formulae. To balance equations on your own, follow these simple rules: Balance a chemical equation when given the unbalanced. the key to balancing chemical equations. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. the simplest and most generally useful method. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free Explain Balancing Equations explain the roles of subscripts and coefficients in chemical equations. Check that all the formulae. the key to balancing chemical equations. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in. Explain Balancing Equations.
From templatelab.com
49 Balancing Chemical Equations Worksheets [with Answers] Explain Balancing Equations a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. the key to balancing chemical equations. The ultimate. Explain Balancing Equations.
From www.wikihow.com
How to Balance Chemical Equations 10 Steps (with Pictures) Explain Balancing Equations a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. Balance a chemical equation when given the unbalanced. explain the roles of subscripts and coefficients in chemical equations. the key to balancing chemical equations. the simplest and most. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Equations PowerPoint Presentation, free download ID Explain Balancing Equations To balance equations on your own, follow these simple rules: the key to balancing chemical equations. a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free Explain Balancing Equations the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. Check that all the formulae. a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. the key to balancing chemical equations. . Explain Balancing Equations.
From www.youtube.com
C + H2 = CH4 Balancing Equations YouTube Explain Balancing Equations a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the.. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Equations PowerPoint Presentation, free download ID Explain Balancing Equations Balance a chemical equation when given the unbalanced. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. the key to balancing chemical equations. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. the simplest and most generally useful. Explain Balancing Equations.
From www.expii.com
Balancing Chemical Equations — Overview & Examples Expii Explain Balancing Equations To balance equations on your own, follow these simple rules: explain the roles of subscripts and coefficients in chemical equations. the key to balancing chemical equations. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. Balance a chemical. Explain Balancing Equations.
From www.slideserve.com
PPT 7.3 Balancing Redox Reactions Using Oxidation Numbers Explain Balancing Equations Balance a chemical equation when given the unbalanced. explain the roles of subscripts and coefficients in chemical equations. a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the.. Explain Balancing Equations.
From www.chemicals.co.uk
Simple Steps to Balance Chemical Equations The Chemistry Blog Explain Balancing Equations a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. Balance a chemical equation when given the unbalanced. . Explain Balancing Equations.
From www.wikihow.com
How to Balance Chemical Equations 11 Steps (with Pictures) Explain Balancing Equations a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. explain the roles of subscripts and coefficients in chemical equations. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. The ultimate goal for balancing. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free Explain Balancing Equations a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. To balance equations on your own, follow these simple rules: Check that all. Explain Balancing Equations.
From kaylyngokehayes.blogspot.com
Chemical Equations Must Be Balanced Because Explain Balancing Equations a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. Check that all the formulae. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. the key to balancing chemical equations. a balanced. Explain Balancing Equations.
From mrbarnestc.com
Balancing Equations Mr Barnes Teaches Chemistry Explain Balancing Equations To balance equations on your own, follow these simple rules: The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. a balanced chemical equation tells you the amounts of reactants and. Explain Balancing Equations.
From learningesferaltf.z21.web.core.windows.net
How To Do Balancing Equations Explain Balancing Equations explain the roles of subscripts and coefficients in chemical equations. the key to balancing chemical equations. To balance equations on your own, follow these simple rules: a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. Check that all. Explain Balancing Equations.
From slideplayer.com
Balancing Equations and Identifying Reaction Types ppt download Explain Balancing Equations The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. Balance a chemical equation when given the unbalanced. To balance equations on your own, follow these simple rules: a balanced chemical. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Equations PowerPoint Presentation, free download ID Explain Balancing Equations the simplest and most generally useful method for balancing chemical equations is called “balancing by inspection,” also known as trial and. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. a balanced chemical equation tells you the amounts. Explain Balancing Equations.
From www.slideserve.com
PPT Balancing Equations PowerPoint Presentation, free download ID Explain Balancing Equations a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. a balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the. Check that all the formulae. Balance. Explain Balancing Equations.
From www.onlinemathlearning.com
Balance Chemical Equations (solutions, examples, videos) Explain Balancing Equations To balance equations on your own, follow these simple rules: a balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both. explain the roles of subscripts and coefficients in chemical equations. a balanced equation is an equation for a chemical reaction in which the. Explain Balancing Equations.
From www.physicsclassroom.com
Balancing Chemical Equations Help Explain Balancing Equations Check that all the formulae. a balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the. To balance equations on your own, follow these simple rules: a balanced equation is a chemical. Explain Balancing Equations.