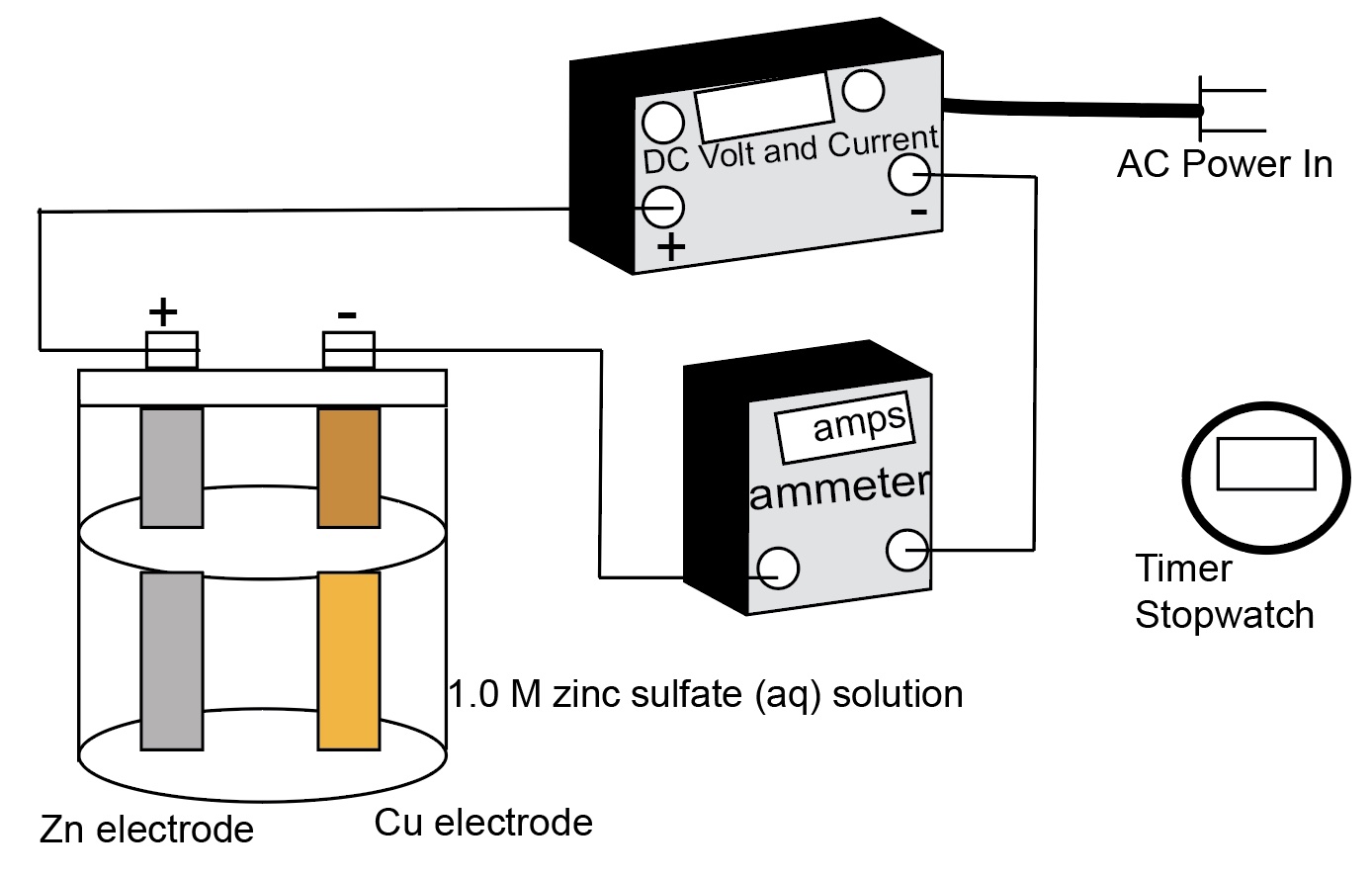
Zinc Electrode In A Cell . that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Then a zinc electrode is placed in the. Then a zinc electrode is placed in the zinc sulfate solution. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from. a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution; Thus, making the metal electrode negatively charged. Then a zinc electrode is placed in the zinc sulfate. This kind of cell includes the galvanic,. a zinc sulfate solution is floated on top of the copper sulfate solution;
from cider.uoregon.edu
that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Then a zinc electrode is placed in the. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from. Then a zinc electrode is placed in the zinc sulfate. Thus, making the metal electrode negatively charged. a zinc sulfate solution is floated on top of the copper sulfate solution; This kind of cell includes the galvanic,. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction.
Electrolysis Cell Demonstration Plating Zinc on Copper CIDER
Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Then a zinc electrode is placed in the. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic,. Then a zinc electrode is placed in the zinc sulfate. a zinc sulfate solution is floated on top of the copper sulfate solution; that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Thus, making the metal electrode negatively charged. a zinc sulfate solution is floated on top of the copper sulfate solution; if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from. Then a zinc electrode is placed in the zinc sulfate solution. a zinc sulfate solution is floated on top of the copper sulfate solution;
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution; Thus, making the metal electrode negatively charged. This kind of cell includes the galvanic,. if we connect the zinc and copper by means of a metallic conductor, the excess electrons that. Zinc Electrode In A Cell.
From www.mdpi.com
Molecules Free FullText Zinc Electrode Cycling in Deep Eutectic Solvent Electrolytes An Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. Then a zinc electrode is placed in the. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. a zinc. Zinc Electrode In A Cell.
From kunduz.com
[ANSWERED] A working electrochemical cell Ecell 0 is formed by zinc and Kunduz Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Thus, making the metal electrode negatively charged. if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from. Then a zinc electrode is. Zinc Electrode In A Cell.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Zinc Electrode In A Cell Thus, making the metal electrode negatively charged. Then a zinc electrode is placed in the. Then a zinc electrode is placed in the zinc sulfate solution. a zinc sulfate solution is floated on top of the copper sulfate solution; This kind of cell includes the galvanic,. an electrochemical cell is a device that produces an electric current from. Zinc Electrode In A Cell.
From www.numerade.com
In the electrochemical cell on the right, the left halfcell is set up with a zinc electrode in Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. This kind of cell includes the galvanic,. a zinc sulfate solution is floated on top of the copper sulfate solution; an electrochemical cell is a device that produces an electric current from energy released. Zinc Electrode In A Cell.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Then a zinc electrode is placed in the zinc sulfate solution. Thus, making the metal electrode negatively charged.. Zinc Electrode In A Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; This kind of cell includes the galvanic,. Then a zinc electrode is placed in the. a zinc sulfate solution is floated on top of the copper sulfate solution; that is, diagrams would show current flowing through an external circuit from the left electrode to. Zinc Electrode In A Cell.
From www.quora.com
In a zinccopper electrolytic cell, is there a build up of zinc atoms on the copper electrode Zinc Electrode In A Cell an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. that is, diagrams would show current flowing through an external circuit from the left electrode. Zinc Electrode In A Cell.
From worksheetlisttabor.z21.web.core.windows.net
Voltaic Cell Worksheet Zinc Electrode In A Cell an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from. a zinc sulfate solution is floated on top of the copper sulfate solution;. Zinc Electrode In A Cell.
From www.researchgate.net
Cross sectional Schematic of a zinc electrowinning cellModel... Download Scientific Diagram Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; This kind of cell includes the galvanic,. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the. if we connect the zinc and copper by means of a metallic conductor, the excess electrons. Zinc Electrode In A Cell.
From www.acs.org
Columbia Dry Cell Battery Landmark American Chemical Society Zinc Electrode In A Cell an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic,. that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. a zinc sulfate solution is floated on top of the. Zinc Electrode In A Cell.
From saylordotorg.github.io
Electrochemistry Zinc Electrode In A Cell Thus, making the metal electrode negatively charged. This kind of cell includes the galvanic,. a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution; if we connect the zinc and copper by means of a metallic conductor, the excess electrons that. Zinc Electrode In A Cell.
From www.numerade.com
A Daniell cell consists of a zinc electrode in 1.00 L of 1.00 M ZnSO4 and a Cu electrode in 1.00 Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution; if we connect the zinc and copper by means. Zinc Electrode In A Cell.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Zinc Electrode In A Cell an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Then a zinc electrode is placed in the zinc sulfate solution. This kind of cell includes the galvanic,. that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in.. Zinc Electrode In A Cell.
From exovfnkcg.blob.core.windows.net
Zinc Electrode Is Placed In at Conrad French blog Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. a zinc sulfate solution is floated on top of the copper sulfate solution; This kind. Zinc Electrode In A Cell.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Then a zinc electrode is placed in the. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. Thus, making the metal electrode. Zinc Electrode In A Cell.
From classmediaunbendable.z21.web.core.windows.net
Electrolysis Of Zinc Iodide Zinc Electrode In A Cell Then a zinc electrode is placed in the. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Then a zinc electrode is placed. Zinc Electrode In A Cell.
From stock.adobe.com
The Daniell cell is a type of electrochemical cell consisting of a container divided into two Zinc Electrode In A Cell Then a zinc electrode is placed in the zinc sulfate. Then a zinc electrode is placed in the. Then a zinc electrode is placed in the zinc sulfate solution. that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Thus, making the metal electrode negatively charged. an. Zinc Electrode In A Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Chemistry Review Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Thus, making the metal electrode negatively charged. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. Then a zinc electrode is placed in. Zinc Electrode In A Cell.
From cider.uoregon.edu
Electrolysis Cell Demonstration Plating Zinc on Copper CIDER Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. This kind of cell includes the galvanic,. that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. if we connect the zinc. Zinc Electrode In A Cell.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in... Download Scientific Diagram Zinc Electrode In A Cell an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Then a zinc electrode is placed in the zinc sulfate solution. a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution; . Zinc Electrode In A Cell.
From www.chemistrystudent.com
Electrochemical Cells (ALevel) ChemistryStudent Zinc Electrode In A Cell Then a zinc electrode is placed in the zinc sulfate. Then a zinc electrode is placed in the zinc sulfate solution. Thus, making the metal electrode negatively charged. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic,. Then a zinc electrode is. Zinc Electrode In A Cell.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation, free download ID4794642 Zinc Electrode In A Cell This kind of cell includes the galvanic,. Then a zinc electrode is placed in the zinc sulfate solution. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous. Zinc Electrode In A Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from. that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode,. Zinc Electrode In A Cell.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; Thus, making the metal electrode negatively charged. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. if we connect the zinc and copper by means of a metallic conductor,. Zinc Electrode In A Cell.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Zinc Electrode In A Cell Then a zinc electrode is placed in the zinc sulfate. a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution; if we connect the zinc and copper. Zinc Electrode In A Cell.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Zinc Electrode In A Cell Then a zinc electrode is placed in the zinc sulfate solution. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution; . Zinc Electrode In A Cell.
From www.researchgate.net
Fibrous zinc electrode for zincair batteries. (a) A rod form for an AA... Download Scientific Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the. a zinc sulfate solution is floated on top of the copper sulfate solution;. Zinc Electrode In A Cell.
From www.nature.com
Effect of electrolyte flow on a gas evolution electrode Scientific Reports Zinc Electrode In A Cell an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from. Then a zinc electrode is placed in the. a zinc sulfate solution is. Zinc Electrode In A Cell.
From app.pandai.org
Standard Electrode Potential Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. Then a zinc electrode is placed in the zinc sulfate. a zinc sulfate solution is floated on top of the copper sulfate solution; a zinc sulfate solution is floated on top of the copper sulfate solution;. Zinc Electrode In A Cell.
From byjus.com
Daniell Cell Definition, Construction & Working with Cell Reactions Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Then a zinc electrode is placed in the zinc sulfate solution. that is, diagrams would show current flowing through an external circuit from the left. Zinc Electrode In A Cell.
From www.youtube.com
What is the potential of a halfcell consisting of zinc electrode in 0.01m ZnSO_4 solution at 25 Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. This kind of cell includes the galvanic,. a zinc sulfate solution is floated on top of the copper sulfate solution; if we connect the zinc and copper by means of a metallic conductor, the excess electrons. Zinc Electrode In A Cell.
From www.toppr.com
26. (a) A cell is prepared by dipping a zinc rod in 1M zinc sulphate solution and a silver 5 Zinc Electrode In A Cell a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the. a zinc sulfate solution is floated on top of the copper sulfate solution; This kind of cell includes the galvanic,. Thus, making the metal electrode negatively charged. Then a zinc electrode is placed in the zinc sulfate. Zinc Electrode In A Cell.
From www.flickriver.com
Copper and Zinc Electrodes in a cell a photo on Flickriver Zinc Electrode In A Cell that is, diagrams would show current flowing through an external circuit from the left electrode to the right electrode, while in. a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate. Then a zinc electrode is placed in the. if we connect the zinc. Zinc Electrode In A Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc Electrode In A Cell Then a zinc electrode is placed in the. a zinc sulfate solution is floated on top of the copper sulfate solution; an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Thus, making the metal electrode negatively charged. a zinc sulfate solution is floated on top of the. Zinc Electrode In A Cell.