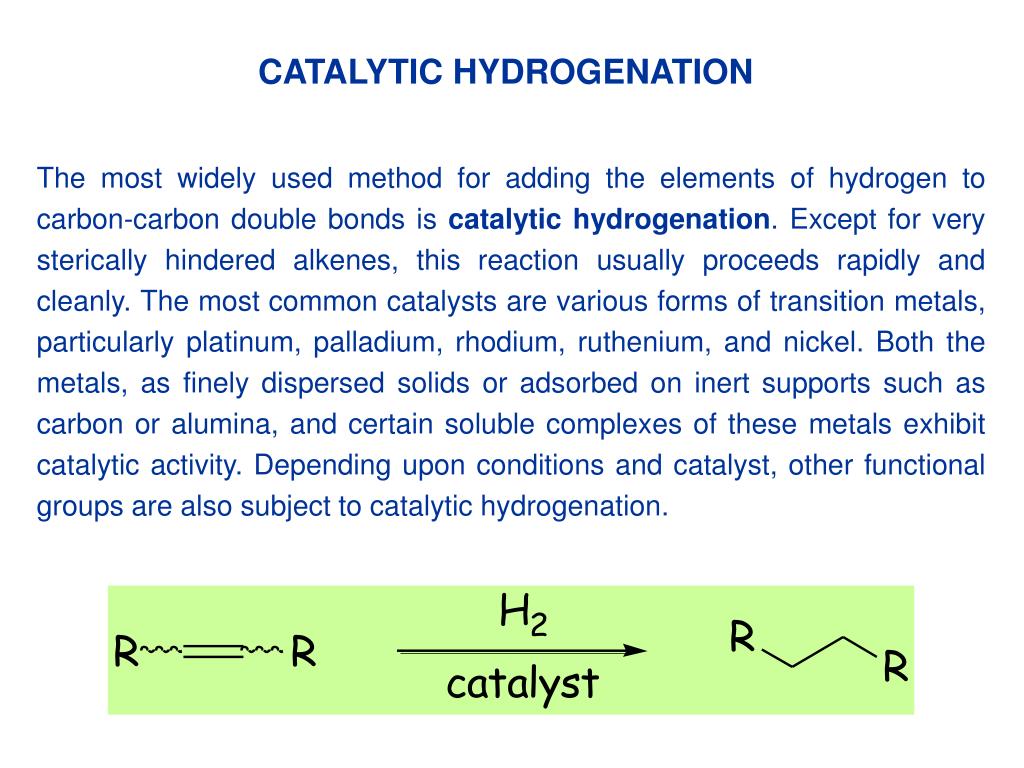
Catalyst In Hydrogenation Reactions . Finely divided metals, such as platinum,. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Common catalysts used are insoluble metals such as. Electrocatalytic hydrogenation (ech) using water as the. Both alkenes and alkynes will readily undergo catalytic hydrogenation. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products.
from www.slideserve.com
Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Electrocatalytic hydrogenation (ech) using water as the. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Finely divided metals, such as platinum,. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Common catalysts used are insoluble metals such as. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the.
PPT CATALYTIC HYDROGENATION PowerPoint Presentation, free download
Catalyst In Hydrogenation Reactions Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Common catalysts used are insoluble metals such as. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Finely divided metals, such as platinum,. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Electrocatalytic hydrogenation (ech) using water as the.
From www.youtube.com
25.02 Hydrogenation of Alkenes YouTube Catalyst In Hydrogenation Reactions Both alkenes and alkynes will readily undergo catalytic hydrogenation. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Catalysts act by lowering the activation energy. Catalyst In Hydrogenation Reactions.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst In Hydrogenation Reactions Common catalysts used are insoluble metals such as. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Finely divided metals, such as platinum,. Both alkenes. Catalyst In Hydrogenation Reactions.
From www.teachoo.com
[Carbon Class 10] Hydrogenation of oils Definition, Reaction Catalyst In Hydrogenation Reactions For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Finely divided metals, such as platinum,. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Electrocatalytic. Catalyst In Hydrogenation Reactions.
From www.slideserve.com
PPT Reactions of Alkenes PowerPoint Presentation, free download ID Catalyst In Hydrogenation Reactions Electrocatalytic hydrogenation (ech) using water as the. Common catalysts used are insoluble metals such as. Finely divided metals, such as platinum,. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Catalytic hydrogenations are important and widely applied. Catalyst In Hydrogenation Reactions.
From www.mdpi.com
Catalysts Free FullText Hydrogenation of Aqueous Acetic Acid over Catalyst In Hydrogenation Reactions Finely divided metals, such as platinum,. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Catalysts act by lowering the activation energy of reactions, but. Catalyst In Hydrogenation Reactions.
From www.chegg.com
Solved 1) (2 points) Predict the products of the Catalyst In Hydrogenation Reactions Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Electrocatalytic hydrogenation (ech) using water as the. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry.. Catalyst In Hydrogenation Reactions.
From commons.wikimedia.org
FileCatalitic cycle for hydrogenation with Wilkinson's catalyst.svg Catalyst In Hydrogenation Reactions Electrocatalytic hydrogenation (ech) using water as the. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Although the. Catalyst In Hydrogenation Reactions.
From www.youtube.com
Alkyne Reduction Hydrogenation, Lindar's catalyst, Dissolving Metal Catalyst In Hydrogenation Reactions Finely divided metals, such as platinum,. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Catalytic. Catalyst In Hydrogenation Reactions.
From chemistry.stackexchange.com
organic chemistry Hydrogenation stereochemistryPd,Pt, Ni catalysts Catalyst In Hydrogenation Reactions Common catalysts used are insoluble metals such as. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Finely divided metals, such as platinum,. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Both alkenes. Catalyst In Hydrogenation Reactions.
From www.science.org
Hydrogenation of carboxylic acids with a homogeneous cobalt catalyst Catalyst In Hydrogenation Reactions Finely divided metals, such as platinum,. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. For hydrogenation processes in a solvent with a solid supported. Catalyst In Hydrogenation Reactions.
From www.slideserve.com
PPT 13. Hydrogenation reactions PowerPoint Presentation, free Catalyst In Hydrogenation Reactions Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Electrocatalytic hydrogenation (ech) using water as the. Although the hydrogenation of an alkene is a thermodynamically favorable reaction,. Catalyst In Hydrogenation Reactions.
From www.researchgate.net
Reaction pathway of CO2 hydrogenation to methanol over CeOx catalysts Catalyst In Hydrogenation Reactions Finely divided metals, such as platinum,. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Common catalysts used are insoluble metals such as. Electrocatalytic hydrogenation (ech) using water as the. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy. Catalyst In Hydrogenation Reactions.
From www.slideserve.com
PPT 7. Alkenes Reactions and Synthesis PowerPoint Presentation, free Catalyst In Hydrogenation Reactions Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Common catalysts used are insoluble metals such as. Finely divided metals, such as platinum,.. Catalyst In Hydrogenation Reactions.
From www.researchgate.net
Scheme 1. Catalytic hydrogenation (a) activating gaseous H 2 as a Catalyst In Hydrogenation Reactions Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Common catalysts used are insoluble metals such as. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Both alkenes and alkynes will readily. Catalyst In Hydrogenation Reactions.
From www.researchgate.net
Catalytic hydrogenation reactions by AGPd aerogel and commercial Catalyst In Hydrogenation Reactions Both alkenes and alkynes will readily undergo catalytic hydrogenation. Common catalysts used are insoluble metals such as. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Finely divided metals, such as platinum,. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not. Catalyst In Hydrogenation Reactions.
From www.researchgate.net
Scheme 1. Hydrogenation reactions using a SAGPd catalyst. Download Catalyst In Hydrogenation Reactions For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Finely divided metals, such as platinum,. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Common catalysts used are insoluble metals such as. Catalysts act. Catalyst In Hydrogenation Reactions.
From sj.jst.go.jp
Hydrogenation of aromatic rings 30x acceleration of reaction rate in Catalyst In Hydrogenation Reactions For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Finely divided metals, such as platinum,. Common catalysts used are insoluble metals such as. Catalysts act. Catalyst In Hydrogenation Reactions.
From www.mdpi.com
Catalysts Free FullText Effect of Oxide Supports on the Activity Catalyst In Hydrogenation Reactions Common catalysts used are insoluble metals such as. Electrocatalytic hydrogenation (ech) using water as the. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Finely divided metals, such as platinum,. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. For hydrogenation processes in a solvent with a solid. Catalyst In Hydrogenation Reactions.
From espanol.libretexts.org
9.11 Reducción de Alquenos Hidrogenación Catalítica LibreTexts Español Catalyst In Hydrogenation Reactions Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Both alkenes and alkynes will readily undergo catalytic hydrogenation. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Catalytic hydrogenations are important and widely applied. Catalyst In Hydrogenation Reactions.
From chemistryscore.com
Hydrogenation of Alkenes ChemistryScore Catalyst In Hydrogenation Reactions Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Electrocatalytic hydrogenation (ech) using water as the. Finely divided metals, such as platinum,. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Both alkenes and. Catalyst In Hydrogenation Reactions.
From www.youtube.com
Catalytic Hydrogenation Reaction of Ethene YouTube Catalyst In Hydrogenation Reactions For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Common catalysts used are insoluble metals such as. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Catalytic hydrogenations are important and widely applied processes for the. Catalyst In Hydrogenation Reactions.
From encyclopedia.pub
IonExchange Resins Application to Hydrogenation Reactions Catalyst In Hydrogenation Reactions Common catalysts used are insoluble metals such as. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Finely divided metals, such as platinum,. Catalysts act by lowering the activation energy of reactions, but they do not change the relative. Catalyst In Hydrogenation Reactions.
From emergencydentistry.com
Hydrogenation Palladium On Carbon Outlet Catalyst In Hydrogenation Reactions Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Electrocatalytic hydrogenation (ech) using water as the.. Catalyst In Hydrogenation Reactions.
From www.slideserve.com
PPT Synthesis of 2º Alcohols PowerPoint Presentation, free download Catalyst In Hydrogenation Reactions Electrocatalytic hydrogenation (ech) using water as the. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Common catalysts used are insoluble metals such as. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Catalytic. Catalyst In Hydrogenation Reactions.
From www.slideserve.com
PPT Reactions of Alkenes PowerPoint Presentation, free download ID Catalyst In Hydrogenation Reactions Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the. Catalyst In Hydrogenation Reactions.
From pubs.acs.org
Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway Catalyst In Hydrogenation Reactions Common catalysts used are insoluble metals such as. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Finely. Catalyst In Hydrogenation Reactions.
From www.mdpi.com
Catalysts Free FullText Hydrogenation of Aqueous Acetic Acid over Catalyst In Hydrogenation Reactions Electrocatalytic hydrogenation (ech) using water as the. Both alkenes and alkynes will readily undergo catalytic hydrogenation. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Common catalysts used are insoluble metals such as. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed. Catalyst In Hydrogenation Reactions.
From www.slideserve.com
PPT CATALYTIC HYDROGENATION PowerPoint Presentation, free download Catalyst In Hydrogenation Reactions Finely divided metals, such as platinum,. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Common catalysts used are insoluble metals such as. Electrocatalytic hydrogenation. Catalyst In Hydrogenation Reactions.
From www.chemistrylearner.com
Hydrogenation Definition, Examples, and Applications Catalyst In Hydrogenation Reactions Common catalysts used are insoluble metals such as. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Both alkenes and alkynes will readily undergo catalytic hydrogenation. Finely divided metals, such as platinum,. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without. Catalyst In Hydrogenation Reactions.
From chemistnotes.com
Catalytic Hydrogenation Mechanism and Application Chemistry Notes Catalyst In Hydrogenation Reactions For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Common catalysts used are insoluble metals such as. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Electrocatalytic hydrogenation (ech) using water as the. Finely. Catalyst In Hydrogenation Reactions.
From chem.unc.edu
Photocatalytic Transfer Hydrogenation in Water Insight into Mechanism Catalyst In Hydrogenation Reactions Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Electrocatalytic hydrogenation (ech) using water as the. Finely divided metals, such as platinum,. Catalysts act by lowering the. Catalyst In Hydrogenation Reactions.
From www.masterorganicchemistry.com
Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes Catalyst In Hydrogenation Reactions Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Electrocatalytic hydrogenation (ech) using water as the. Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Both alkenes and alkynes will readily undergo catalytic hydrogenation.. Catalyst In Hydrogenation Reactions.
From www.researchgate.net
Visualization of the reaction mechanism of the direct hydrogenation of Catalyst In Hydrogenation Reactions Electrocatalytic hydrogenation (ech) using water as the. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Both alkenes and alkynes will readily undergo catalytic hydrogenation. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved is the reduction of the. Finely divided. Catalyst In Hydrogenation Reactions.
From www.masterorganicchemistry.com
Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes Catalyst In Hydrogenation Reactions Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. For hydrogenation processes in a solvent with a solid supported metal catalyst, an important process involved. Catalyst In Hydrogenation Reactions.
From www.researchgate.net
Scheme 1. Hydrogenation of aliphatic alkenes using NiC450 catalyst Catalyst In Hydrogenation Reactions Catalytic hydrogenations are important and widely applied processes for the reduction of organic compounds both in academic laboratories and in industry. Common catalysts used are insoluble metals such as. Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst. Catalysts act by lowering the activation energy of reactions, but. Catalyst In Hydrogenation Reactions.