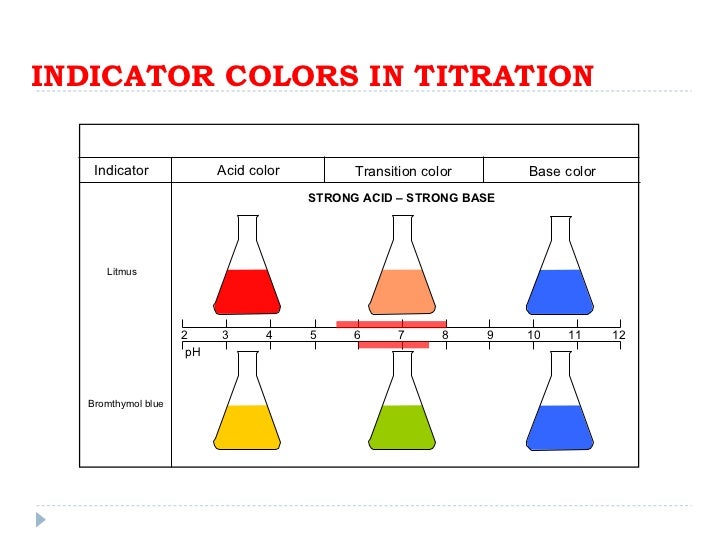
Indicators For Strong Base Weak Acid Titration . Compute sample ph at important stages of a titration. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. | titrations flow chart | determine the k a or k b. Explain the function of acid. Chemists are typically interested in calculating volume and acidity data for the following critical points: This page assumes that you know.
from mungfali.com
Compute sample ph at important stages of a titration. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. | titrations flow chart | determine the k a or k b. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Chemists are typically interested in calculating volume and acidity data for the following critical points: The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Explain the function of acid. This page assumes that you know.
Acid Base Titration Indicator
Indicators For Strong Base Weak Acid Titration This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Chemists are typically interested in calculating volume and acidity data for the following critical points: | titrations flow chart | determine the k a or k b. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Explain the function of acid. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know. Compute sample ph at important stages of a titration. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.
From www.writework.com
Titration of amino acids WriteWork Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical. Indicators For Strong Base Weak Acid Titration.
From slideplayer.com
Weak Acid Strong Base Titration ppt download Indicators For Strong Base Weak Acid Titration | titrations flow chart | determine the k a or k b. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid. Indicators For Strong Base Weak Acid Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicators For Strong Base Weak Acid Titration The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Compute sample ph at important stages of a titration. Explain the function of acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments.. Indicators For Strong Base Weak Acid Titration.
From slidetodoc.com
ACID BASE TITRATION INDICATORS Titration a method of Indicators For Strong Base Weak Acid Titration Explain the function of acid. Compute sample ph at important stages of a titration. This page assumes that you know. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. | titrations flow chart | determine the k a or k b. Chemists are typically interested. Indicators For Strong Base Weak Acid Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a. Indicators For Strong Base Weak Acid Titration.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Indicators For Strong Base Weak Acid Titration Explain the function of acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Chemists are typically interested in calculating volume and acidity data for the following critical points: This page assumes that you know. Compute sample ph at important stages of a titration. | titrations flow. Indicators For Strong Base Weak Acid Titration.
From philschatz.com
AcidBase Titrations · Chemistry Indicators For Strong Base Weak Acid Titration This page assumes that you know. Compute sample ph at important stages of a titration. Chemists are typically interested in calculating volume and acidity data for the following critical points: Explain the function of acid. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base. Indicators For Strong Base Weak Acid Titration.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Indicators For Strong Base Weak Acid Titration | titrations flow chart | determine the k a or k b. Chemists are typically interested in calculating volume and acidity data for the following critical points: Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. The titration of a. Indicators For Strong Base Weak Acid Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Indicators For Strong Base Weak Acid Titration Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Compute sample ph at important stages of a titration. Chemists are typically interested in calculating volume and acidity data for the following critical points: The titration of a weak acid with a strong base involves the direct transfer. Indicators For Strong Base Weak Acid Titration.
From www.vrogue.co
Weak Acid Vs Strong Base Titration Curve vrogue.co Indicators For Strong Base Weak Acid Titration Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. This page assumes that you know. Explain the function of acid. | titrations flow chart | determine the k a or k b. Chemists are typically interested in calculating volume and acidity data for the following critical points:. Indicators For Strong Base Weak Acid Titration.
From webmis.highland.cc.il.us
AcidBase Titrations Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide. Indicators For Strong Base Weak Acid Titration.
From mungfali.com
Acid Base Titration Indicator Indicators For Strong Base Weak Acid Titration Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. This page assumes that you know. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. This figure shows. Indicators For Strong Base Weak Acid Titration.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Explain the function of acid. This page assumes that you know. | titrations flow chart | determine the k a or k b.. Indicators For Strong Base Weak Acid Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicators For Strong Base Weak Acid Titration The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Chemists are typically interested in calculating volume and acidity data for the following critical points: | titrations flow chart | determine the k a or k b. Ph indicators are weak acids or weak bases that. Indicators For Strong Base Weak Acid Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Indicators For Strong Base Weak Acid Titration Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. The titration of a weak acid with a strong. Indicators For Strong Base Weak Acid Titration.
From saylordotorg.github.io
AcidBase Titrations Indicators For Strong Base Weak Acid Titration This page assumes that you know. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. | titrations flow chart | determine the k a or k b. Compute sample ph at important stages of a titration. Determine ph and concentrations of chemical species at any point in. Indicators For Strong Base Weak Acid Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Indicators For Strong Base Weak Acid Titration This page assumes that you know. Compute sample ph at important stages of a titration. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Chemists are typically interested in calculating volume and. Indicators For Strong Base Weak Acid Titration.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Indicators For Strong Base Weak Acid Titration The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Compute sample ph at important stages of a titration. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Explain the function of acid.. Indicators For Strong Base Weak Acid Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: This page assumes that you know. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. | titrations. Indicators For Strong Base Weak Acid Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Indicators For Strong Base Weak Acid Titration Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Chemists are typically interested in calculating volume and acidity data for the following critical points: Compute sample ph at important stages of a titration. Explain the function of acid. This figure. Indicators For Strong Base Weak Acid Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicators For Strong Base Weak Acid Titration Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Explain the function of acid. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid). Indicators For Strong Base Weak Acid Titration.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记5.2.6 Titration Curves翰林国际教育 Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Explain the function of acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the. Indicators For Strong Base Weak Acid Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID2134619 Indicators For Strong Base Weak Acid Titration This page assumes that you know. Compute sample ph at important stages of a titration. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Chemists are typically interested in calculating volume and acidity data for the following critical points: This figure shows plots of ph. Indicators For Strong Base Weak Acid Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Indicators For Strong Base Weak Acid Titration Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Compute sample ph at important stages of a titration. This page assumes that you. Indicators For Strong Base Weak Acid Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicators For Strong Base Weak Acid Titration Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid. Indicators For Strong Base Weak Acid Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Indicators For Strong Base Weak Acid Titration Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. | titrations flow chart | determine the k a or k b. Compute sample ph at important stages of a titration. Determine ph and concentrations of chemical species at any point in the titration of a weak acid. Indicators For Strong Base Weak Acid Titration.
From www.vrogue.co
Weak Acid Vs Strong Base Titration Curve vrogue.co Indicators For Strong Base Weak Acid Titration This page assumes that you know. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Chemists are typically. Indicators For Strong Base Weak Acid Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: Explain the function of acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Determine ph and concentrations of chemical species at any point in the titration of a weak. Indicators For Strong Base Weak Acid Titration.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Indicators For Strong Base Weak Acid Titration Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. | titrations flow chart | determine the k a or k b. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a. Indicators For Strong Base Weak Acid Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Indicators For Strong Base Weak Acid Titration Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. This figure shows plots of ph versus volume of. Indicators For Strong Base Weak Acid Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Explain the function of acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the. Indicators For Strong Base Weak Acid Titration.
From www.vrogue.co
Strong Acid And Strong Base Titration Curve vrogue.co Indicators For Strong Base Weak Acid Titration This page assumes that you know. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Compute sample ph at important stages of a titration. Chemists are typically interested in calculating volume and acidity data for the following critical points: |. Indicators For Strong Base Weak Acid Titration.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Indicators For Strong Base Weak Acid Titration Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. This page assumes that you know. Explain the function of acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or. Indicators For Strong Base Weak Acid Titration.
From www.slideshare.net
Acid base titration Indicators For Strong Base Weak Acid Titration This page assumes that you know. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Chemists. Indicators For Strong Base Weak Acid Titration.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 Indicators For Strong Base Weak Acid Titration Chemists are typically interested in calculating volume and acidity data for the following critical points: Compute sample ph at important stages of a titration. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. Indicators For Strong Base Weak Acid Titration.