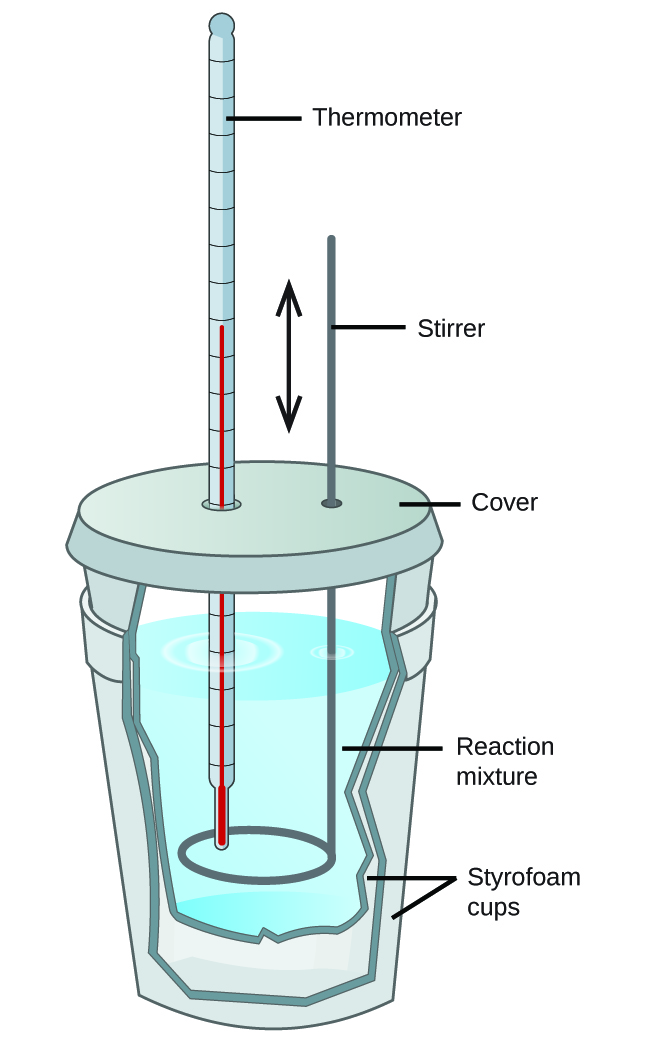
Explain How A Calorimeter Works . A calorimeter has two vessels: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. An outer vessel and an inner vessel. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid.
from wisc.pb.unizin.org
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter has two vessels: A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. An outer vessel and an inner vessel. Apply the first law of thermodynamics to calorimetry.
5.2 Calorimetry Chemistry
Explain How A Calorimeter Works A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. A calorimeter has two vessels: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. An outer vessel and an inner vessel. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic reaction occurs in solution in a. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Explain How A Calorimeter Works An outer vessel and an inner vessel. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter has two vessels: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. Compare heat flow. Explain How A Calorimeter Works.
From www.coursehero.com
[Solved] 1. Explain how a calorimeter works, and how you... Course Hero Explain How A Calorimeter Works For example, when an exothermic reaction occurs in solution in a. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. An outer vessel and an inner vessel. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Explain How A Calorimeter Works.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Explain How A Calorimeter Works Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. An outer vessel and an inner vessel. A calorimeter has two vessels: A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. A calorimeter. Explain How A Calorimeter Works.
From www.youtube.com
050 Calorimetry YouTube Explain How A Calorimeter Works A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. For example, when an exothermic reaction occurs in solution in a. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first. Explain How A Calorimeter Works.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Explain How A Calorimeter Works For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter has two vessels: A calorimeter measures the mass of liquid and the temperature change. Explain How A Calorimeter Works.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure Explain How A Calorimeter Works Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. For example, when an exothermic reaction occurs in solution in a. A calorimeter measures the. Explain How A Calorimeter Works.
From www.youtube.com
Principle of Calorimetry YouTube Explain How A Calorimeter Works A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. For example, when an exothermic. Calorimeter, device for. Explain How A Calorimeter Works.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Explain How A Calorimeter Works A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. An outer vessel and an inner vessel. A calorimeter has two vessels: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or. Explain How A Calorimeter Works.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Explain How A Calorimeter Works Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter has two vessels: An outer vessel and an inner vessel. For example, when an exothermic reaction occurs in solution in a. Apply the first law of thermodynamics to calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid. Explain How A Calorimeter Works.
From ddscalorimeters.com
How does a bomb calorimeter work? Explain How A Calorimeter Works An outer vessel and an inner vessel. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter measures the mass of liquid and the temperature change. Explain How A Calorimeter Works.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Explain How A Calorimeter Works Apply the first law of thermodynamics to calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. For example, when an exothermic reaction occurs in solution in a. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Explain How A Calorimeter Works.
From www.coursehero.com
[Solved] 1. Explain how a calorimeter works, and how you... Course Hero Explain How A Calorimeter Works A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. An outer vessel and an inner vessel. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to. Explain How A Calorimeter Works.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Explain How A Calorimeter Works Apply the first law of thermodynamics to calorimetry. An outer vessel and an inner vessel. For example, when an exothermic. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Explain How A Calorimeter Works.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Explain How A Calorimeter Works Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. An outer vessel and an inner vessel. A calorimeter has two vessels: Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Compare heat flow. Explain How A Calorimeter Works.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Explain How A Calorimeter Works Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. An outer vessel and an inner vessel. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Explain How A Calorimeter Works.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Explain How A Calorimeter Works Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. An outer. Explain How A Calorimeter Works.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Explain How A Calorimeter Works A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. For example, when an exothermic. For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Explain How A Calorimeter Works.
From www.researchgate.net
Schematic diagram of the calorimeter used in this work Download Explain How A Calorimeter Works For example, when an exothermic reaction occurs in solution in a. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. For example, when an exothermic. A calorimeter is a. Explain How A Calorimeter Works.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Explain How A Calorimeter Works For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. A calorimeter. Explain How A Calorimeter Works.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Explain How A Calorimeter Works Apply the first law of thermodynamics to calorimetry. A calorimeter has two vessels: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic reaction occurs in. Explain How A Calorimeter Works.
From www.thoughtco.com
Calorimeter Definition in Chemistry Explain How A Calorimeter Works Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter has two vessels: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on. Explain How A Calorimeter Works.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Explain How A Calorimeter Works Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. A calorimeter is a device used to measure the amount. Explain How A Calorimeter Works.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Explain How A Calorimeter Works Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic reaction occurs in solution in a. An outer vessel and an inner vessel. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. Explain How A Calorimeter Works.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Explain How A Calorimeter Works An outer vessel and an inner vessel. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter has two vessels: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. Apply the first. Explain How A Calorimeter Works.
From ddscalorimeters.com
How does a bomb calorimeter work? Explain How A Calorimeter Works A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. An outer vessel and an inner vessel. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry measures enthalpy. Explain How A Calorimeter Works.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Explain How A Calorimeter Works For example, when an exothermic. An outer vessel and an inner vessel. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost. Explain How A Calorimeter Works.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Explain How A Calorimeter Works Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. For example, when an exothermic. Compare heat flow from hot. Explain How A Calorimeter Works.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Explain How A Calorimeter Works Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. Calorimetry measures enthalpy. Explain How A Calorimeter Works.
From www.animalia-life.club
Calorimeter Diagram Explain How A Calorimeter Works Apply the first law of thermodynamics to calorimetry. An outer vessel and an inner vessel. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. For example, when an exothermic reaction occurs in solution in a. A calorimeter measures the mass of liquid and the. Explain How A Calorimeter Works.
From www.numerade.com
SOLVEDExplain how calorimetry works to calculate ΔH or ΔE for a Explain How A Calorimeter Works A calorimeter has two vessels: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter measures the mass of liquid and the temperature change. Explain How A Calorimeter Works.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Explain How A Calorimeter Works An outer vessel and an inner vessel. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. A calorimeter. Explain How A Calorimeter Works.
From users.highland.edu
Calorimetry Explain How A Calorimeter Works Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter has two vessels: A calorimeter is a device used to measure the amount. Explain How A Calorimeter Works.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog Explain How A Calorimeter Works A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. A calorimeter is a device used to measure the. Explain How A Calorimeter Works.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Explain How A Calorimeter Works A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Explain How A Calorimeter Works.
From klayjstec.blob.core.windows.net
How Calorimeter Function at Geneva Katzman blog Explain How A Calorimeter Works An outer vessel and an inner vessel. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. A calorimeter measures the mass of liquid and the temperature. Explain How A Calorimeter Works.