Does Water Evaporate In Hot Oil . Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. Oil doesn’t mix with water, and most oils are less dense than water. Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. The heat built up by you driving for an hour would be enough for the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. However, the oil layer can slow the. What this means is that when you drop water into hot oil it evaporates pretty much instantly. Liquid water is made up of molecules of h 2 o. Some oils evaporate themselves, though, and if you. The temperature of the oil is above the boiling point. Water does not have to reach the boiling point to evaporate. So oil floats on top of water, and usually makes a. How does water evaporate without boiling? When liquid water meets dry air, it is not in equilibrium;
from www.iqsdirectory.com
Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Some oils evaporate themselves, though, and if you. What this means is that when you drop water into hot oil it evaporates pretty much instantly. Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. However, the oil layer can slow the. The temperature of the oil is above the boiling point. Water does not have to reach the boiling point to evaporate. So oil floats on top of water, and usually makes a. When liquid water meets dry air, it is not in equilibrium;
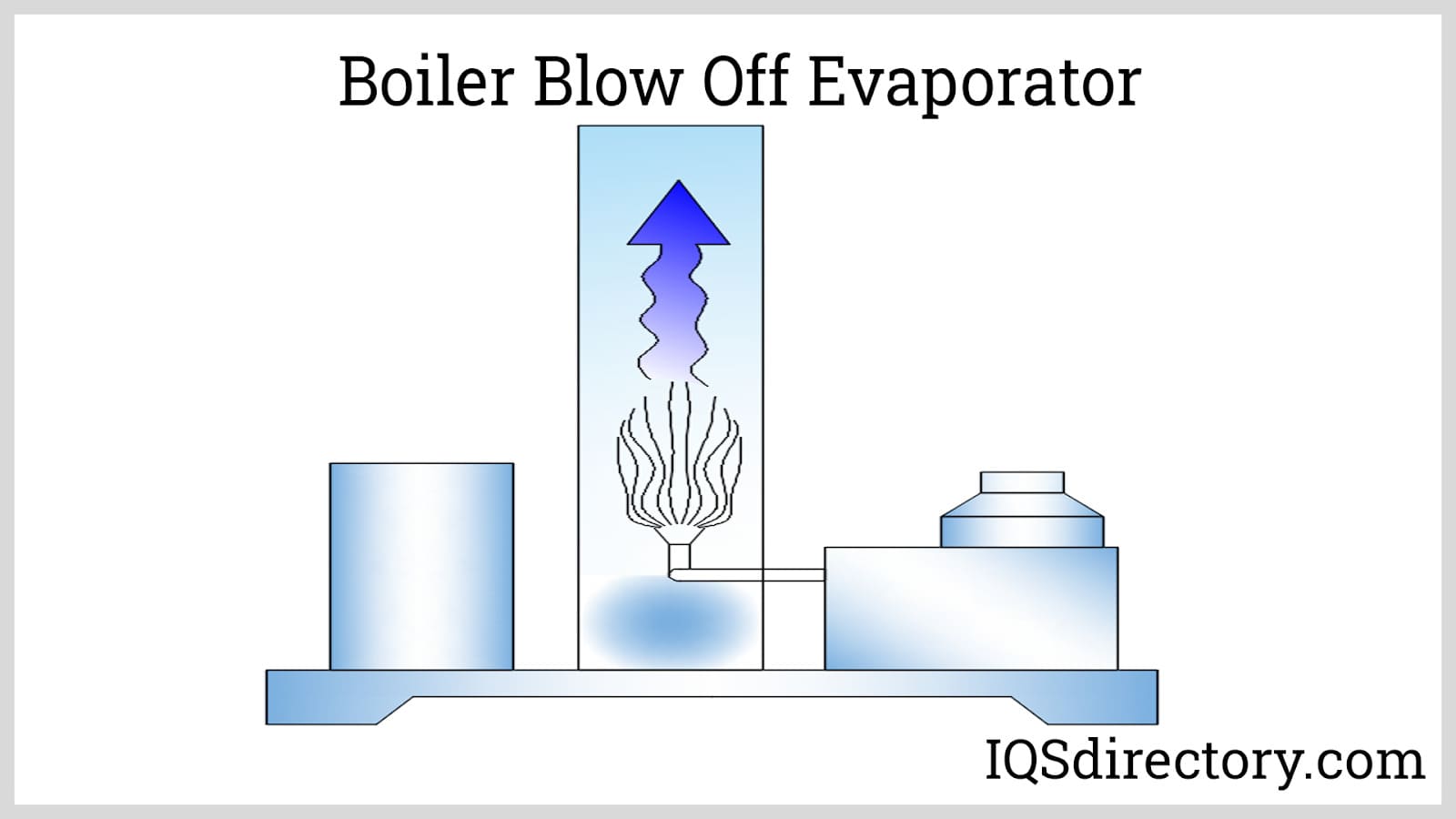
Wastewater Evaporator What Is It? How Does It Work? Types
Does Water Evaporate In Hot Oil When liquid water meets dry air, it is not in equilibrium; Liquid water is made up of molecules of h 2 o. How does water evaporate without boiling? Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. The heat built up by you driving for an hour would be enough for the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. When liquid water meets dry air, it is not in equilibrium; Oil doesn’t mix with water, and most oils are less dense than water. However, the oil layer can slow the. Water does not have to reach the boiling point to evaporate. What this means is that when you drop water into hot oil it evaporates pretty much instantly. The temperature of the oil is above the boiling point. Some oils evaporate themselves, though, and if you. So oil floats on top of water, and usually makes a. Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil.
From hottubsreport.com
All about Hot Tub Evaporation? Hot Tubs Report Does Water Evaporate In Hot Oil Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. However, the oil layer can slow the. Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. Liquid water is made up of molecules of h 2 o. Some oils evaporate themselves, though, and if you. The. Does Water Evaporate In Hot Oil.
From www.atmo.arizona.edu
Saturating the air with water vapor Does Water Evaporate In Hot Oil The heat built up by you driving for an hour would be enough for the. When liquid water meets dry air, it is not in equilibrium; Some oils evaporate themselves, though, and if you. What this means is that when you drop water into hot oil it evaporates pretty much instantly. Water does not have to reach the boiling point. Does Water Evaporate In Hot Oil.
From shinestarpk.blogspot.com
Education World What Is The Evaporation? Does Water Evaporate In Hot Oil Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. How does water evaporate without boiling? Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. However, the oil layer can slow the. Some oils evaporate themselves, though, and if you. Water does not. Does Water Evaporate In Hot Oil.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Does Water Evaporate In Hot Oil When liquid water meets dry air, it is not in equilibrium; Oil doesn’t mix with water, and most oils are less dense than water. Some oils evaporate themselves, though, and if you. The temperature of the oil is above the boiling point. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure. Does Water Evaporate In Hot Oil.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Does Water Evaporate In Hot Oil When liquid water meets dry air, it is not in equilibrium; However, the oil layer can slow the. Oil doesn’t mix with water, and most oils are less dense than water. Water does not have to reach the boiling point to evaporate. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure. Does Water Evaporate In Hot Oil.
From how-things-work-science-projects.com
How do Temperature and Wind affect Evaporation? How Things Work Does Water Evaporate In Hot Oil Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. Water does not have to reach the boiling point to evaporate. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Liquid water is made up of molecules of h 2 o. Oil. Does Water Evaporate In Hot Oil.
From masterconceptsinchemistry.com
How does the vapor pressure of solution depend on the concentration of Does Water Evaporate In Hot Oil The heat built up by you driving for an hour would be enough for the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The temperature of the oil is above the boiling point. Water does not have to reach the boiling point to evaporate. Some oils evaporate themselves,. Does Water Evaporate In Hot Oil.
From www.nsta.org
Q What’s the difference between evaporation and boiling? NSTA Does Water Evaporate In Hot Oil How does water evaporate without boiling? The heat built up by you driving for an hour would be enough for the. When liquid water meets dry air, it is not in equilibrium; What this means is that when you drop water into hot oil it evaporates pretty much instantly. Some oils evaporate themselves, though, and if you. Oil doesn’t mix. Does Water Evaporate In Hot Oil.
From www.bbc.co.uk
Evaporation BBC Bitesize Does Water Evaporate In Hot Oil When liquid water meets dry air, it is not in equilibrium; Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Liquid water is made up of molecules of h 2 o. However, the. Does Water Evaporate In Hot Oil.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Does Water Evaporate In Hot Oil Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water does not have to reach the boiling point to evaporate. How does water evaporate without boiling? When liquid water meets dry air, it. Does Water Evaporate In Hot Oil.
From www.slideshare.net
Evaporation Does Water Evaporate In Hot Oil Water does not have to reach the boiling point to evaporate. The temperature of the oil is above the boiling point. Liquid water is made up of molecules of h 2 o. Oil doesn’t mix with water, and most oils are less dense than water. Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the. Does Water Evaporate In Hot Oil.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Does Water Evaporate In Hot Oil Some oils evaporate themselves, though, and if you. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. How does water evaporate without boiling? Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. So oil floats on top of water, and usually. Does Water Evaporate In Hot Oil.
From foodwine.com
Does Oil Evaporate When Cooking Food & Wine Does Water Evaporate In Hot Oil Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. How does water evaporate without boiling? So oil floats on top of water, and usually makes a. The heat built up by you driving for an hour. Does Water Evaporate In Hot Oil.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Does Water Evaporate In Hot Oil Liquid water is made up of molecules of h 2 o. Water does not have to reach the boiling point to evaporate. The heat built up by you driving for an hour would be enough for the. However, the oil layer can slow the. Some oils evaporate themselves, though, and if you. How does water evaporate without boiling? What this. Does Water Evaporate In Hot Oil.
From www.metoffice.gov.uk
Water cycle Met Office Does Water Evaporate In Hot Oil Water does not have to reach the boiling point to evaporate. However, the oil layer can slow the. So oil floats on top of water, and usually makes a. The heat built up by you driving for an hour would be enough for the. Water molecules evaporate off the surface until the amount of water in the air creates enough. Does Water Evaporate In Hot Oil.
From www.meer.com
How does water evaporate? Meer Does Water Evaporate In Hot Oil The heat built up by you driving for an hour would be enough for the. Some oils evaporate themselves, though, and if you. What this means is that when you drop water into hot oil it evaporates pretty much instantly. The temperature of the oil is above the boiling point. Liquid water is made up of molecules of h 2. Does Water Evaporate In Hot Oil.
From vehicleanswers.com
Does Engine Oil Evaporate? Vehicle Answers Does Water Evaporate In Hot Oil Some oils evaporate themselves, though, and if you. Water does not have to reach the boiling point to evaporate. However, the oil layer can slow the. The heat built up by you driving for an hour would be enough for the. Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. Water molecules. Does Water Evaporate In Hot Oil.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Does Water Evaporate In Hot Oil However, the oil layer can slow the. Water does not have to reach the boiling point to evaporate. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The temperature of the oil is above the boiling point. The heat built up by you driving for an hour would be. Does Water Evaporate In Hot Oil.
From www.animalia-life.club
Evaporates Does Water Evaporate In Hot Oil Some oils evaporate themselves, though, and if you. Liquid water is made up of molecules of h 2 o. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. However, the oil layer. Does Water Evaporate In Hot Oil.
From www.researchgate.net
Evaporation and film boiling process of water droplets sprayed on the Does Water Evaporate In Hot Oil Oil doesn’t mix with water, and most oils are less dense than water. The temperature of the oil is above the boiling point. The heat built up by you driving for an hour would be enough for the. What this means is that when you drop water into hot oil it evaporates pretty much instantly. Some oils evaporate themselves, though,. Does Water Evaporate In Hot Oil.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Does Water Evaporate In Hot Oil The temperature of the oil is above the boiling point. When liquid water meets dry air, it is not in equilibrium; How does water evaporate without boiling? Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water does not have to reach the boiling point to evaporate. Oil doesn’t. Does Water Evaporate In Hot Oil.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Water Evaporate In Hot Oil So oil floats on top of water, and usually makes a. Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. Oil doesn’t mix with water, and most oils are less dense than water. What this means is that when you drop water into hot oil it evaporates pretty much instantly. However, the oil. Does Water Evaporate In Hot Oil.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube Does Water Evaporate In Hot Oil Oil doesn’t mix with water, and most oils are less dense than water. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water does not have to reach the boiling point to evaporate. However, the oil layer can slow the. Actually, the water can evaporate at lower temperatures than. Does Water Evaporate In Hot Oil.
From www.spicysaltysweet.com
Does Oil Evaporate? Busting The Myth Spicy Salty Sweet Does Water Evaporate In Hot Oil The heat built up by you driving for an hour would be enough for the. Water dissolves very little in ordinary oil, so hardly any water molecules diffuse through the oil. Oil doesn’t mix with water, and most oils are less dense than water. How does water evaporate without boiling? Some oils evaporate themselves, though, and if you. The temperature. Does Water Evaporate In Hot Oil.
From exoojivaj.blob.core.windows.net
Does Water Evaporate When Cooking at Spencer Gilligan blog Does Water Evaporate In Hot Oil The temperature of the oil is above the boiling point. Liquid water is made up of molecules of h 2 o. The heat built up by you driving for an hour would be enough for the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. So oil floats on. Does Water Evaporate In Hot Oil.
From www.snexplores.org
Explainer What are the different states of matter? Does Water Evaporate In Hot Oil What this means is that when you drop water into hot oil it evaporates pretty much instantly. However, the oil layer can slow the. Some oils evaporate themselves, though, and if you. Oil doesn’t mix with water, and most oils are less dense than water. Water molecules evaporate off the surface until the amount of water in the air creates. Does Water Evaporate In Hot Oil.
From bigtimekitchen.com
Does Water Evaporate Faster With Or Without A Lid? Does Water Evaporate In Hot Oil Oil doesn’t mix with water, and most oils are less dense than water. Water does not have to reach the boiling point to evaporate. So oil floats on top of water, and usually makes a. Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. How does water evaporate without boiling? The heat. Does Water Evaporate In Hot Oil.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Does Water Evaporate In Hot Oil When liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The temperature of the oil is above the boiling point. How does water evaporate without boiling? So oil floats on top of water, and usually makes a. Some oils. Does Water Evaporate In Hot Oil.
From www.pinterest.com
Changes of states evaporation water boiling vector image on Does Water Evaporate In Hot Oil The temperature of the oil is above the boiling point. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water does not have to reach the boiling point to evaporate. Actually, the water can evaporate at lower temperatures than 100°c, just as it could with no oil. When liquid. Does Water Evaporate In Hot Oil.
From www.researchgate.net
Schematic illustration of solardriven interfacial water evaporation Does Water Evaporate In Hot Oil Oil doesn’t mix with water, and most oils are less dense than water. How does water evaporate without boiling? So oil floats on top of water, and usually makes a. Water does not have to reach the boiling point to evaporate. The heat built up by you driving for an hour would be enough for the. The temperature of the. Does Water Evaporate In Hot Oil.
From www.scienceabc.com
Are Evaporation And Boiling The Same? » Science ABC Does Water Evaporate In Hot Oil How does water evaporate without boiling? Some oils evaporate themselves, though, and if you. The temperature of the oil is above the boiling point. What this means is that when you drop water into hot oil it evaporates pretty much instantly. Liquid water is made up of molecules of h 2 o. When liquid water meets dry air, it is. Does Water Evaporate In Hot Oil.
From schematicparttod.z21.web.core.windows.net
Why Does Oil Not Evaporate Does Water Evaporate In Hot Oil The temperature of the oil is above the boiling point. However, the oil layer can slow the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Liquid water is made up of molecules of h 2 o. What this means is that when you drop water into hot oil. Does Water Evaporate In Hot Oil.
From www.teachoo.com
Why is Evaporation called a surface phenomenon? Teachoo Does Water Evaporate In Hot Oil What this means is that when you drop water into hot oil it evaporates pretty much instantly. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. When liquid water meets dry air, it is not in equilibrium; However, the oil layer can slow the. Actually, the water can evaporate. Does Water Evaporate In Hot Oil.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo Does Water Evaporate In Hot Oil How does water evaporate without boiling? Some oils evaporate themselves, though, and if you. The temperature of the oil is above the boiling point. Oil doesn’t mix with water, and most oils are less dense than water. When liquid water meets dry air, it is not in equilibrium; However, the oil layer can slow the. So oil floats on top. Does Water Evaporate In Hot Oil.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Does Water Evaporate In Hot Oil The heat built up by you driving for an hour would be enough for the. So oil floats on top of water, and usually makes a. However, the oil layer can slow the. Water does not have to reach the boiling point to evaporate. How does water evaporate without boiling? What this means is that when you drop water into. Does Water Evaporate In Hot Oil.