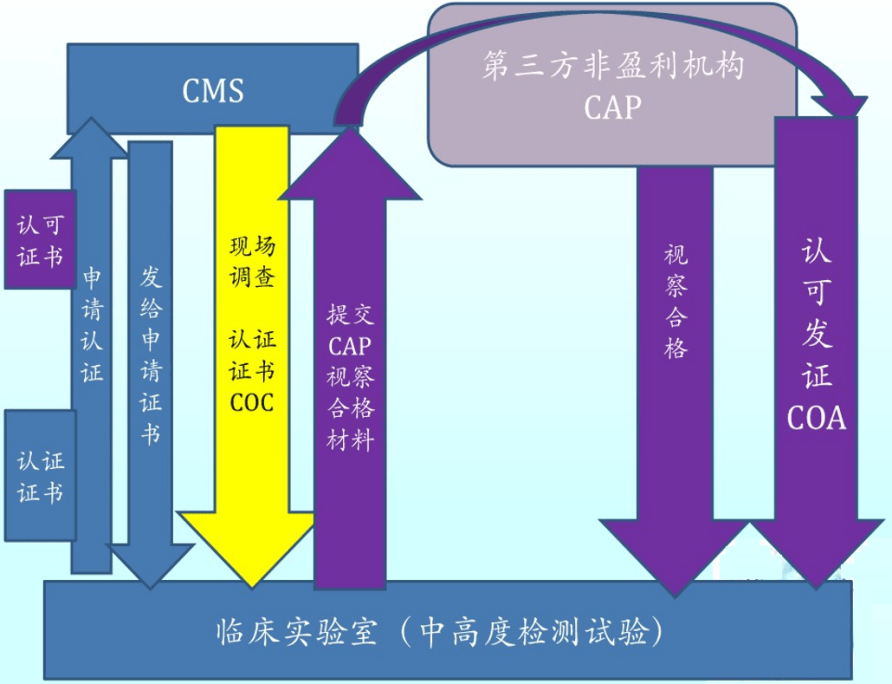
What Is Cap Clia . The law establishes standards for all united states. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Clia is a regulatory program that sets. Cap laboratory accreditation helps laboratories: Meet required standards from clia, fda and. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services. Maintain accuracy of test results and ensure accurate patient diagnosis; The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality.
from www.sohu.com
Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services. Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Maintain accuracy of test results and ensure accurate patient diagnosis; Clia is a regulatory program that sets. Cap laboratory accreditation helps laboratories: Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. The law establishes standards for all united states. We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality.
终于搞懂了!实验室的CLIA、CAP、ISO15189,NCCL到底是什么_认证_临床_检验
What Is Cap Clia Cap laboratory accreditation helps laboratories: We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality. Maintain accuracy of test results and ensure accurate patient diagnosis; Cap laboratory accreditation helps laboratories: Meet required standards from clia, fda and. The law establishes standards for all united states. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Clia is a regulatory program that sets. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services.
From www.prolisphere.com
What is CLIA compliance, and why do you need it? What Is Cap Clia Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Clia is a regulatory program that sets. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Maintain accuracy of test results and ensure accurate patient diagnosis; The clinical laboratory improvement. What Is Cap Clia.
From www.prolisphere.com
What is CLIA Certification and How do I get CLIA certification? What Is Cap Clia Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality. The law establishes standards for all united states. Understanding the similarities and differences of the clinical laboratory improvement amendments. What Is Cap Clia.
From www.youtube.com
CAP Competency Assessment Program What is CLIA Competency Assessment What Is Cap Clia Cap laboratory accreditation helps laboratories: Meet required standards from clia, fda and. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality. Understanding the similarities and differences of the. What Is Cap Clia.
From www.psomagen.com
CAP/CLIA Certifications Psomagen What Is Cap Clia Cap laboratory accreditation helps laboratories: Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. The law establishes standards for all united states. We outline the differences between clia, cap & cola regulations & accreditations, and what they. What Is Cap Clia.
From cellcarta.com
Obtaining CAP/CLIA Accreditation in a Canadian Clinical CRO What Is Cap Clia The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Meet required standards from clia, fda and. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure. What Is Cap Clia.
From genomicspersonalizedhealth.com
CLIA/CAP Labs Genomics Personalized Health What Is Cap Clia Maintain accuracy of test results and ensure accurate patient diagnosis; Clia is a regulatory program that sets. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. The law establishes standards for all united states. Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Clia stands for clinical. What Is Cap Clia.
From www.prolisphere.com
What is CLIA Certification and How do I get CLIA certification? What Is Cap Clia Meet required standards from clia, fda and. The law establishes standards for all united states. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Maintain accuracy of test results and ensure accurate patient diagnosis; Clia is a regulatory program that sets. Understanding the similarities and differences of the clinical laboratory improvement amendments. What Is Cap Clia.
From sapient.bio
Sapient’s High Complexity CLIA Lab Receives CAP Accreditation What Is Cap Clia Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Meet required standards from clia, fda and. Cap laboratory accreditation helps laboratories: Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Maintain accuracy of test results and ensure accurate patient. What Is Cap Clia.
From www.sohu.com
经验分享:实验室认证认可之:CLIA和CAP简介_临床 What Is Cap Clia Maintain accuracy of test results and ensure accurate patient diagnosis; Meet required standards from clia, fda and. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. The law establishes standards for all united states. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in. What Is Cap Clia.
From www.diacarta.com
Clia Lab About Us DiaCarta, Inc. What Is Cap Clia Clia is a regulatory program that sets. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services. Meet required standards from clia, fda and. We outline the differences. What Is Cap Clia.
From hostagencyreviews.com
What is a CLIA Number? CLIA vs. ARC and CLIA vs. IATA What Is Cap Clia Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Cap laboratory accreditation helps laboratories: We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for. What Is Cap Clia.
From www.sohu.com
经验分享:实验室认证认可之:CLIA和CAP简介_临床 What Is Cap Clia We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality. The law establishes standards for all united states. Clia is a regulatory program that sets. Maintain accuracy of test results and ensure accurate patient diagnosis; Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. The. What Is Cap Clia.
From www.poweredbyash.com
7 Things You Need to Know About CLIA/CAP Lab Certification Blog Ash What Is Cap Clia The law establishes standards for all united states. Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Cap laboratory accreditation helps laboratories: Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure. What Is Cap Clia.
From en.novogene.com
Clinical Whole Exome Sequencing (CLIA/CAP) Novogene What Is Cap Clia Maintain accuracy of test results and ensure accurate patient diagnosis; Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. We outline the differences between clia, cap & cola regulations & accreditations,. What Is Cap Clia.
From gbu-presnenskij.ru
Definition CLIA Certified? CAP Accredited? What Does This, 51 OFF What Is Cap Clia Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Maintain accuracy of test results and ensure accurate patient diagnosis; Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services. We outline the differences between clia, cap & cola regulations &. What Is Cap Clia.
From www.mriglobal.org
CAP/CLIA Laboratory for Quality Testing MRIGlobal What Is Cap Clia Meet required standards from clia, fda and. The law establishes standards for all united states. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Understanding the similarities and differences of the. What Is Cap Clia.
From www.sohu.com
终于搞懂了!实验室的CLIA、CAP、ISO15189,NCCL到底是什么_认证_临床_检验 What Is Cap Clia The law establishes standards for all united states. Meet required standards from clia, fda and. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Maintain accuracy of test results and. What Is Cap Clia.
From www.sohu.com
终于搞懂了!实验室的CLIA、CAP、ISO15189,NCCL到底是什么_认证_临床_检验 What Is Cap Clia Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Clia is a regulatory program that sets. The law establishes standards for all united states. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. The clinical laboratory improvement amendments (clia) of 1988 is a. What Is Cap Clia.
From www.isosh.com
CLIA与CAP 关于临床试验室金标准,您不得不掌握的知识点!公司快讯久顺企管集团4006583933,医疗器械注册,CE认证 What Is Cap Clia Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. The law establishes standards for all united states. Meet required standards from clia, fda and. Cap laboratory accreditation helps laboratories: Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Clia is a regulatory program. What Is Cap Clia.
From www.bioagilytix.com
CLIA, COLA & CAP What’s the Difference? Navigating Regulations What Is Cap Clia The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services. Learn. What Is Cap Clia.
From www.novogene.com
Novogene’s UC Davis CLIA Certified Clinical Lab has passed the CAP’s What Is Cap Clia The law establishes standards for all united states. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality. Cap laboratory accreditation helps laboratories: Clia is a regulatory program that sets. Meet required standards. What Is Cap Clia.
From www.psomagen.com
CAP/CLIA Certifications Psomagen What Is Cap Clia The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Cap laboratory accreditation helps laboratories: Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and. What Is Cap Clia.
From www.darkdaily.com
SPECIAL REPORT Better CLIA Compliance Reviewing Top Deficiencies What Is Cap Clia Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. The law establishes standards for all united states. Cap laboratory accreditation helps laboratories: The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Maintain accuracy of test results and ensure accurate patient. What Is Cap Clia.
From blog.genohub.com
Assessing CLIA / CAP Certified Next Generation Sequencing Facilities What Is Cap Clia Clia is a regulatory program that sets. Meet required standards from clia, fda and. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality. Cap laboratory accreditation helps laboratories: Maintain accuracy of test. What Is Cap Clia.
From www.scribd.com
CLIA CAP Training Digital & Social Media Digital Technology What Is Cap Clia We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. The. What Is Cap Clia.
From www.caplinehealthcaremanagement.com
WHAT IS CLIA IN MEDICAL BILLING? What Is Cap Clia The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Maintain accuracy of test results and ensure accurate patient diagnosis; Clia is a regulatory program that sets. Cap laboratory accreditation helps laboratories: Clia stands for. What Is Cap Clia.
From chekco.com
CAP và CLIA 2 T/C chất lượng hàng đầu phòng xét nghiệm What Is Cap Clia Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. The law establishes standards for all united states. Learn the difference between clia and cap, two types of laboratory accreditation programs. What Is Cap Clia.
From www.psomagen.com
CAP/CLIA Certifications Psomagen What Is Cap Clia Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Meet required standards from clia, fda and. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability. What Is Cap Clia.
From onehealthlabs.com
CAP and CLIA Certifications Why They Matter One Health Labs What Is Cap Clia The law establishes standards for all united states. Meet required standards from clia, fda and. Maintain accuracy of test results and ensure accurate patient diagnosis; Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates. What Is Cap Clia.
From www.psomagen.com
CAP/CLIA Certifications Psomagen What Is Cap Clia Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Maintain accuracy of test results and ensure accurate patient diagnosis; The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. We outline the differences between clia, cap & cola regulations & accreditations, and what they. What Is Cap Clia.
From www.psomagen.com
CAP/CLIA Certifications Psomagen What Is Cap Clia Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Meet required standards from clia, fda and. Cap laboratory accreditation helps laboratories: Clia is a regulatory program that sets. Clia stands for clinical laboratory improvement amendments, a law that. What Is Cap Clia.
From www.geneoscopy.com
Geneoscopy Receives Accreditation from the College of American What Is Cap Clia Maintain accuracy of test results and ensure accurate patient diagnosis; Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. Understanding the similarities and differences of the clinical laboratory improvement amendments. What Is Cap Clia.
From www.clickonlaboratory.com
Understanding CLIA, COLA & CAP What do they mean? What Is Cap Clia Learn how to apply for and maintain cap accreditation, a globally recognized standard for laboratory quality and compliance. The clinical laboratory improvement amendments (clia) of 1988 is a federal law that regulates clinical laboratory testing. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. We outline the differences. What Is Cap Clia.
From www.poweredbyash.com
Why CLIA/CAP Certification Matters Blog Ash Wellness What Is Cap Clia Cap laboratory accreditation helps laboratories: Understanding the similarities and differences of the clinical laboratory improvement amendments (clia) and the. Learn the difference between clia and cap, two types of laboratory accreditation programs that ensure quality and reliability in lab services. The law establishes standards for all united states. The clinical laboratory improvement amendments (clia) of 1988 is a federal law. What Is Cap Clia.
From www.sohu.com
经验分享:实验室认证认可之:CLIA和CAP简介_临床 What Is Cap Clia The law establishes standards for all united states. Clia stands for clinical laboratory improvement amendments, a law that regulates all clinical laboratory testing on humans in the us, except. Maintain accuracy of test results and ensure accurate patient diagnosis; We outline the differences between clia, cap & cola regulations & accreditations, and what they mean for laboratory testing quality. Cap. What Is Cap Clia.