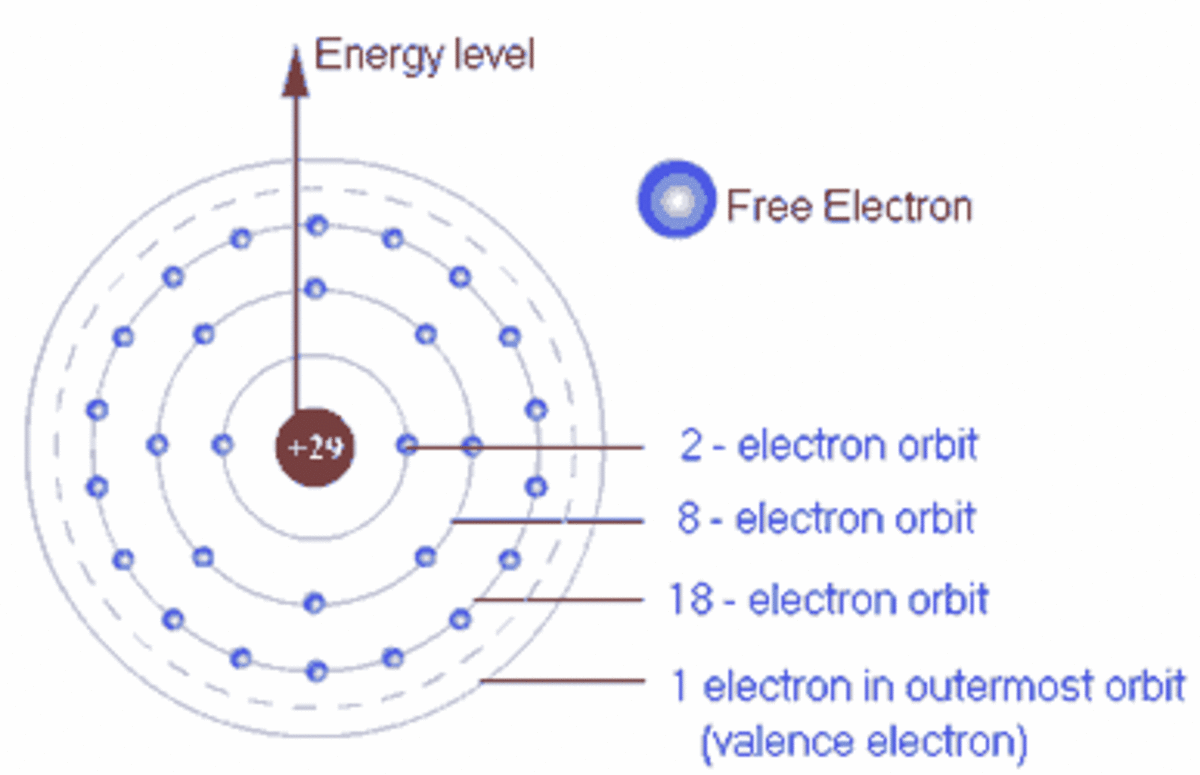
Copper Electrons In Outer Shell . the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. The valence electrons of an atom are the electrons in its outermost shell, and they. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. valence electrons and valency of copper. But for most of the. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical.
from owlcation.com
The valence electrons of an atom are the electrons in its outermost shell, and they. valence electrons and valency of copper. But for most of the. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n.
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together? Owlcation
Copper Electrons In Outer Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. The valence electrons of an atom are the electrons in its outermost shell, and they. But for most of the. valence electrons and valency of copper.
From www.pngegg.com
Atom illustration, Bohr model Sodium Atom Chemistry Rutherford model, copper shell, chemical Copper Electrons In Outer Shell The valence electrons of an atom are the electrons in its outermost shell, and they. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. valence electrons and valency of copper. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled. Copper Electrons In Outer Shell.
From mungfali.com
How Many Electrons Are In Each Shell Copper Electrons In Outer Shell the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. But for most of the. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. the group 18 atoms helium (he), neon (ne), and argon (ar). Copper Electrons In Outer Shell.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Table of Elements and Chemistry Copper Electrons In Outer Shell The valence electrons of an atom are the electrons in its outermost shell, and they. But for most of the. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron. Copper Electrons In Outer Shell.
From stock.adobe.com
Cu Copper Element Information Facts, Properties, Trends, Uses and comparison Periodic Table of Copper Electrons In Outer Shell But for most of the. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. The valence electrons of an atom are the electrons in its outermost. Copper Electrons In Outer Shell.
From www.slideshare.net
Copper Copper Electrons In Outer Shell the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. valence electrons and valency of copper. the number of electron shells in. Copper Electrons In Outer Shell.
From fyoehimsi.blob.core.windows.net
Copper Outer Orbital Electrons at Christine Meek blog Copper Electrons In Outer Shell the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. But for most of the. how to write the electron configuration for copper. Copper Electrons In Outer Shell.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron configuration of an atom of copper Copper Electrons In Outer Shell the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. valence electrons and valency of copper. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. The valence electrons of an atom are. Copper Electrons In Outer Shell.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electrons In Outer Shell The valence electrons of an atom are the electrons in its outermost shell, and they. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is. Copper Electrons In Outer Shell.
From www.wou.edu
Ch105 Chapter 2 Atoms, Elements and The Periodic Table Chemistry Copper Electrons In Outer Shell The valence electrons of an atom are the electrons in its outermost shell, and they. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. valence. Copper Electrons In Outer Shell.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Electrons In Outer Shell the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. But for most of the. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the group 18 atoms helium (he), neon (ne),. Copper Electrons In Outer Shell.
From cursosonlineweb.com
Configuración electrónica del cobre Cursos Online Copper Electrons In Outer Shell But for most of the. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the group 18 atoms helium (he), neon (ne), and argon (ar). Copper Electrons In Outer Shell.
From www.webelements.com
Elements Periodic Table » Copper » properties of free atoms Copper Electrons In Outer Shell valence electrons and valency of copper. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. But for most of the. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. the. Copper Electrons In Outer Shell.
From giogesxfy.blob.core.windows.net
Copper Number Of Electrons Per Shell at Holly Donovan blog Copper Electrons In Outer Shell But for most of the. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. valence electrons and valency of copper. The valence electrons of an atom are the electrons in its outermost shell, and they. how to write the electron configuration. Copper Electrons In Outer Shell.
From www.youtube.com
An ion has 18 electrons in the outermost shell, it is (a) Cu^+ (b) Th^4+ (c) Cs^+ (d) K^+ (1990 Copper Electrons In Outer Shell valence electrons and valency of copper. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. The valence electrons of an atom are the electrons in its outermost shell, and they. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the. Copper Electrons In Outer Shell.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Electrons In Outer Shell the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. The valence electrons of an atom are the electrons in its outermost shell, and. Copper Electrons In Outer Shell.
From www.alamy.com
Symbol and electron diagram of Copper illustration Stock Vector Image & Art Alamy Copper Electrons In Outer Shell the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. The valence electrons of an atom are the electrons in its outermost shell, and they. the number of electron shells in an atom and the distribution of electrons in the shell determine the. Copper Electrons In Outer Shell.
From valenceelectrons.com
How to Find the Valence Electrons for Cobalt (Co)? Copper Electrons In Outer Shell But for most of the. The valence electrons of an atom are the electrons in its outermost shell, and they. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. the number of electron shells in an atom and the distribution of electrons in the shell. Copper Electrons In Outer Shell.
From www.slideshare.net
Structure Of Atoms Part 3 Copper Electrons In Outer Shell But for most of the. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. The valence electrons of an atom are the electrons in its outermost shell, and they. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write. Copper Electrons In Outer Shell.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Electrons In Outer Shell The valence electrons of an atom are the electrons in its outermost shell, and they. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is. Copper Electrons In Outer Shell.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration, chemical data, and Copper Electrons In Outer Shell But for most of the. The valence electrons of an atom are the electrons in its outermost shell, and they. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write. Copper Electrons In Outer Shell.
From mammothmemory.net
Copper atoms Copper Electrons In Outer Shell the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. The valence electrons of an atom are the electrons in its outermost shell, and they. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. the. Copper Electrons In Outer Shell.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electrons In Outer Shell valence electrons and valency of copper. The valence electrons of an atom are the electrons in its outermost shell, and they. But for most of the. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the valence electrons in copper (cu) are the electrons. Copper Electrons In Outer Shell.
From www.schoolmykids.com
Compare Copper vs Carbon Periodic Table Element Comparison Compare Properties, Structure, Facts Copper Electrons In Outer Shell The valence electrons of an atom are the electrons in its outermost shell, and they. But for most of the. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. valence electrons and valency of copper. the valence electrons in copper (cu) are the electrons. Copper Electrons In Outer Shell.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electrons In Outer Shell the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. But for most of the. The valence electrons of an atom are the electrons in its outermost. Copper Electrons In Outer Shell.
From mybios.me
Copper Periodic Table Protons Neutrons And Electrons Bios Pics Copper Electrons In Outer Shell the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. But for most of the. The valence electrons of an atom are the electrons in its outermost shell, and they. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write. Copper Electrons In Outer Shell.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electrons In Outer Shell the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. The valence electrons of an atom are the electrons in its outermost shell, and they. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. the. Copper Electrons In Outer Shell.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper Electrons In Outer Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. The valence electrons of an atom are the electrons in its outermost shell, and. Copper Electrons In Outer Shell.
From www.alamy.com
3d render of atom structure of copper isolated over white background Stock Photo 137220153 Alamy Copper Electrons In Outer Shell valence electrons and valency of copper. The valence electrons of an atom are the electrons in its outermost shell, and they. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. the number of electron shells in an atom and the distribution of electrons in. Copper Electrons In Outer Shell.
From slideplayer.com
International System of Units ppt download Copper Electrons In Outer Shell the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. valence electrons and valency of copper. But for most of the. The valence. Copper Electrons In Outer Shell.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together? Owlcation Copper Electrons In Outer Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. But for most of the. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled outer electron shells, making it unnecessary for them to share electrons. the valence electrons in copper. Copper Electrons In Outer Shell.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Copper Electrons In Outer Shell The valence electrons of an atom are the electrons in its outermost shell, and they. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. But for most of the. valence electrons and valency of copper. how to write the electron configuration for copper (cu, cu+, and cu2+). Copper Electrons In Outer Shell.
From www.pinterest.jp
The copper atom. Copper atom, Electronic schematics, Free energy Copper Electrons In Outer Shell the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. But for most of the. valence electrons and valency of copper. the. Copper Electrons In Outer Shell.
From www.alamy.com
Symbol and electron diagram for Copper illustration Stock Vector Image & Art Alamy Copper Electrons In Outer Shell valence electrons and valency of copper. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The valence electrons of an atom are the electrons in its outermost shell, and they. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled. Copper Electrons In Outer Shell.
From www.allaboutcircuits.com
Valence and Crystal Structure Solidstate Device Theory Electronics Textbook Copper Electrons In Outer Shell The valence electrons of an atom are the electrons in its outermost shell, and they. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. valence electrons and valency of copper. the group 18 atoms helium (he), neon (ne), and argon (ar) all have filled. Copper Electrons In Outer Shell.
From kethewtdudley.blogspot.com
Electronic Configuration of Copper KethewtDudley Copper Electrons In Outer Shell But for most of the. the valence electrons in copper (cu) are the electrons in the outermost shell of the atom, which is the fourth shell (n. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. how to write the electron configuration for copper (cu, cu+, and. Copper Electrons In Outer Shell.