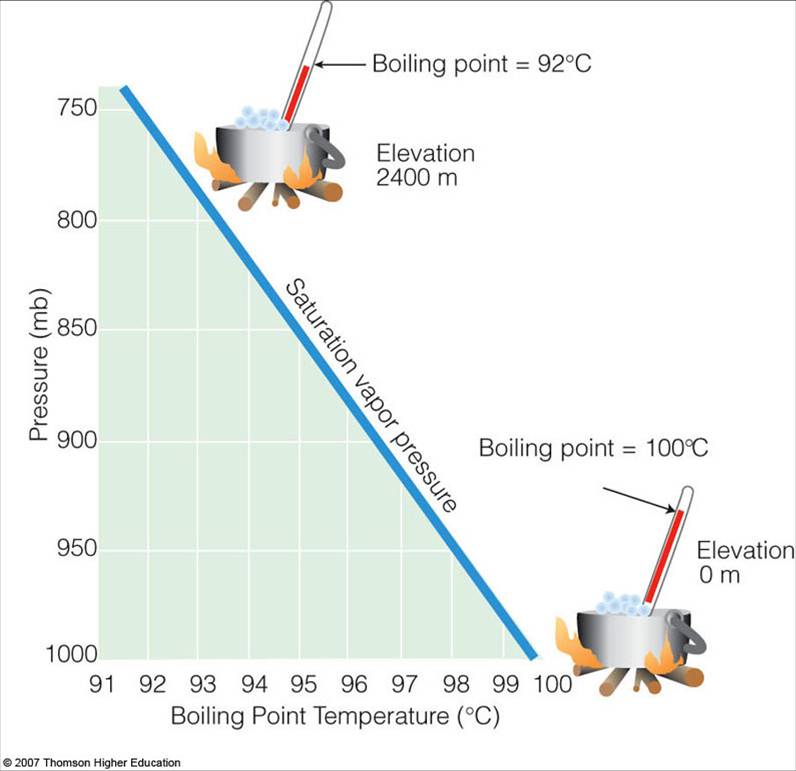
What Is Water's Highest Boiling Point . Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. As the altitude increases, the atmospheric pressure pushing. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. However, in this section we learn that the boiling point of a liquid. The boiling point of a liquid depends on temperature,. The boiling point of a liquid varies according to the applied pressure; It is well known that boiling point depends on temperature. Most know that water boils at 100 degrees celsius.
from apollo.lsc.vsc.edu
The boiling point of a liquid varies according to the applied pressure; For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of a liquid depends on temperature,. As the altitude increases, the atmospheric pressure pushing. Most know that water boils at 100 degrees celsius. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. However, in this section we learn that the boiling point of a liquid. It is well known that boiling point depends on temperature.
Saturation Vapor Pressure and the Boiling Point
What Is Water's Highest Boiling Point Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. It is well known that boiling point depends on temperature. As the altitude increases, the atmospheric pressure pushing. Most know that water boils at 100 degrees celsius. However, in this section we learn that the boiling point of a liquid. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The boiling point of a liquid depends on temperature,. The boiling point of a liquid varies according to the applied pressure;
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Is Water's Highest Boiling Point One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. It is well known that boiling point depends on temperature. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The boiling point of a. What Is Water's Highest Boiling Point.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Is Water's Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; Most know that water boils at 100 degrees celsius. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. It is well known that boiling point depends on temperature. The boiling point of. What Is Water's Highest Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart What Is Water's Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Most know that water boils at 100 degrees celsius. The boiling point of a liquid depends on temperature,. As the altitude increases, the atmospheric pressure pushing. It is. What Is Water's Highest Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is Water's Highest Boiling Point It is well known that boiling point depends on temperature. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. However, in this section we learn that the boiling point of a liquid. One of the most significant changes that occur in high altitude areas. What Is Water's Highest Boiling Point.
From www.numerade.com
SOLVED Highest boiling point Lowest solubility in water Highest What Is Water's Highest Boiling Point For example, for water, the boiling point is 100ºc at a pressure of 1 atm. However, in this section we learn that the boiling point of a liquid. Most know that water boils at 100 degrees celsius. The boiling point of a liquid varies according to the applied pressure; It is well known that boiling point depends on temperature. Water. What Is Water's Highest Boiling Point.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID What Is Water's Highest Boiling Point Most know that water boils at 100 degrees celsius. However, in this section we learn that the boiling point of a liquid. The boiling point of a liquid depends on temperature,. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Water boils at a lower temperature as you gain. What Is Water's Highest Boiling Point.
From slideplayer.com
Properties of Water. ppt download What Is Water's Highest Boiling Point One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. As the altitude increases, the atmospheric pressure pushing. Most know that water boils at 100 degrees celsius. The boiling point of a liquid depends on temperature,. It is well known that boiling point depends on temperature. However, in this section. What Is Water's Highest Boiling Point.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is Water's Highest Boiling Point One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Most know that water boils at 100 degrees celsius. However, in this section we learn that the boiling point of a liquid. The boiling point of a liquid varies according to the applied pressure; The boiling point of a liquid. What Is Water's Highest Boiling Point.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is Water's Highest Boiling Point It is well known that boiling point depends on temperature. As the altitude increases, the atmospheric pressure pushing. The boiling point of a liquid depends on temperature,. The boiling point of a liquid varies according to the applied pressure; Most know that water boils at 100 degrees celsius. However, in this section we learn that the boiling point of a. What Is Water's Highest Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Water's Highest Boiling Point For example, for water, the boiling point is 100ºc at a pressure of 1 atm. As the altitude increases, the atmospheric pressure pushing. The boiling point of a liquid depends on temperature,. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. It is well. What Is Water's Highest Boiling Point.
From www.youtube.com
22 Boiling Point Elevation Explanation YouTube What Is Water's Highest Boiling Point It is well known that boiling point depends on temperature. Most know that water boils at 100 degrees celsius. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. As the altitude increases, the atmospheric pressure pushing. Water boils at a lower temperature as you gain altitude (e.g., going higher. What Is Water's Highest Boiling Point.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Is Water's Highest Boiling Point Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. As the altitude increases, the atmospheric pressure pushing. However, in this section we learn that the boiling point of a liquid. It is well known that boiling point depends on temperature. For example, for water,. What Is Water's Highest Boiling Point.
From www.slideserve.com
PPT Entry Task Nov 27 th Block 1 PowerPoint Presentation, free What Is Water's Highest Boiling Point As the altitude increases, the atmospheric pressure pushing. The boiling point of a liquid depends on temperature,. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. However, in this section we learn that the boiling point of a liquid. For example, for water, the. What Is Water's Highest Boiling Point.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is Water's Highest Boiling Point One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Most know that water boils at 100 degrees celsius. It is well known that boiling. What Is Water's Highest Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is Water's Highest Boiling Point It is well known that boiling point depends on temperature. However, in this section we learn that the boiling point of a liquid. As the altitude increases, the atmospheric pressure pushing. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. One of the most. What Is Water's Highest Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Water's Highest Boiling Point The boiling point of a liquid depends on temperature,. It is well known that boiling point depends on temperature. As the altitude increases, the atmospheric pressure pushing. Most know that water boils at 100 degrees celsius. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. For example, for water,. What Is Water's Highest Boiling Point.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is Water's Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; As the altitude increases, the atmospheric pressure pushing. The boiling point of a liquid depends on temperature,. It is well known that boiling point depends on temperature. Most know that water boils at 100 degrees celsius. Water boils at a lower temperature as you gain altitude (e.g., going. What Is Water's Highest Boiling Point.
From www.youtube.com
Why is the Boiling Point of water (H2O) so high? YouTube What Is Water's Highest Boiling Point Most know that water boils at 100 degrees celsius. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. However, in this section we learn that the boiling point of a liquid. The boiling point of a liquid varies according to the applied pressure; The boiling point of a liquid. What Is Water's Highest Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is Water's Highest Boiling Point It is well known that boiling point depends on temperature. However, in this section we learn that the boiling point of a liquid. As the altitude increases, the atmospheric pressure pushing. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. One of the most. What Is Water's Highest Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Water's Highest Boiling Point As the altitude increases, the atmospheric pressure pushing. The boiling point of a liquid depends on temperature,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. It is well known that boiling point depends. What Is Water's Highest Boiling Point.
From www.slideserve.com
PPT Hydrocarbon Derivatives PowerPoint Presentation, free download What Is Water's Highest Boiling Point It is well known that boiling point depends on temperature. The boiling point of a liquid varies according to the applied pressure; One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. As the altitude increases, the atmospheric pressure pushing. The boiling point of a liquid depends on temperature,. However,. What Is Water's Highest Boiling Point.
From www.slideserve.com
PPT EXAM I Powerpoint II A Little Chemistry PowerPoint Presentation What Is Water's Highest Boiling Point One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. The boiling point of a liquid depends on temperature,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of a liquid varies according to the applied pressure; However, in this section. What Is Water's Highest Boiling Point.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country What Is Water's Highest Boiling Point The boiling point of a liquid depends on temperature,. The boiling point of a liquid varies according to the applied pressure; Most know that water boils at 100 degrees celsius. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. As the altitude increases, the atmospheric pressure pushing. It is. What Is Water's Highest Boiling Point.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Is Water's Highest Boiling Point However, in this section we learn that the boiling point of a liquid. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of a liquid varies according to the applied pressure; As the altitude increases, the atmospheric pressure pushing. Most know that water boils at 100 degrees celsius. One of the. What Is Water's Highest Boiling Point.
From joixgvqec.blob.core.windows.net
Why Is The Boiling Point Of Water High at Tina Edmonds blog What Is Water's Highest Boiling Point As the altitude increases, the atmospheric pressure pushing. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. One of the most significant changes that occur in high altitude areas. What Is Water's Highest Boiling Point.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is Water's Highest Boiling Point Most know that water boils at 100 degrees celsius. The boiling point of a liquid depends on temperature,. As the altitude increases, the atmospheric pressure pushing. It is well known that boiling point depends on temperature. However, in this section we learn that the boiling point of a liquid. For example, for water, the boiling point is 100ºc at a. What Is Water's Highest Boiling Point.
From www.slideserve.com
PPT Water as a Polar Molecule PowerPoint Presentation, free download What Is Water's Highest Boiling Point For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The boiling point of a liquid depends on temperature,. However, in this section we learn that the boiling point of. What Is Water's Highest Boiling Point.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Is Water's Highest Boiling Point The boiling point of a liquid depends on temperature,. Most know that water boils at 100 degrees celsius. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature. What Is Water's Highest Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Water's Highest Boiling Point As the altitude increases, the atmospheric pressure pushing. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. However, in this section we learn that the boiling point of a liquid. The boiling point of a liquid varies according to the applied pressure; The boiling. What Is Water's Highest Boiling Point.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Is Water's Highest Boiling Point It is well known that boiling point depends on temperature. However, in this section we learn that the boiling point of a liquid. The boiling point of a liquid varies according to the applied pressure; Most know that water boils at 100 degrees celsius. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain). What Is Water's Highest Boiling Point.
From joixgvqec.blob.core.windows.net
Why Is The Boiling Point Of Water High at Tina Edmonds blog What Is Water's Highest Boiling Point For example, for water, the boiling point is 100ºc at a pressure of 1 atm. As the altitude increases, the atmospheric pressure pushing. Most know that water boils at 100 degrees celsius. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The boiling point. What Is Water's Highest Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is Water's Highest Boiling Point It is well known that boiling point depends on temperature. The boiling point of a liquid varies according to the applied pressure; Most know that water boils at 100 degrees celsius. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. However, in this section. What Is Water's Highest Boiling Point.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes What Is Water's Highest Boiling Point One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Most know that water boils at 100 degrees celsius. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain). What Is Water's Highest Boiling Point.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is Water's Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; As the altitude increases, the atmospheric pressure pushing. Most know that water boils at 100 degrees celsius. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. For example, for water, the boiling point is 100ºc at a. What Is Water's Highest Boiling Point.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is Water's Highest Boiling Point One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. It is well known that boiling point depends on temperature. The boiling point of a liquid varies according to the applied pressure; As the altitude. What Is Water's Highest Boiling Point.