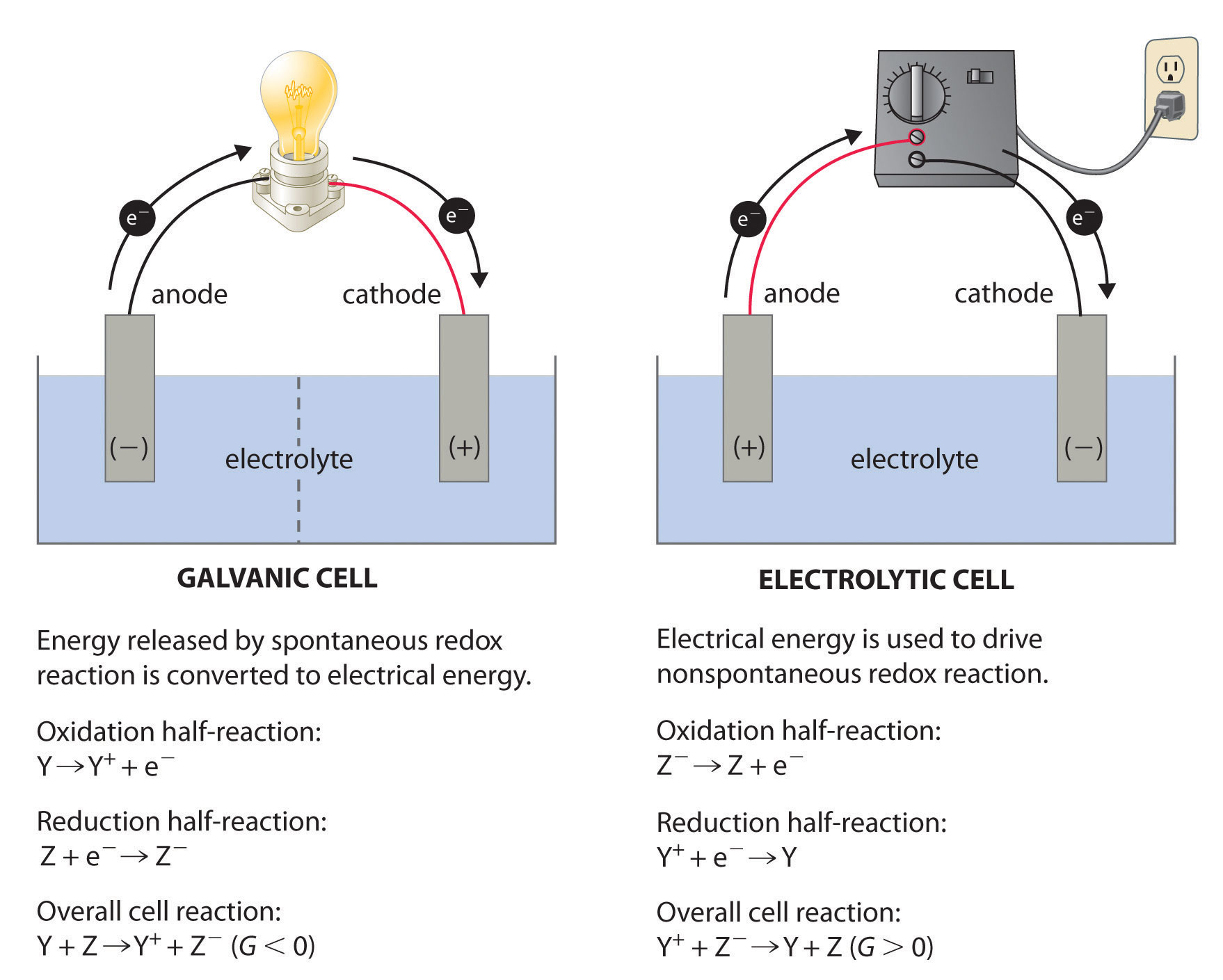
Distinguish Between Galvanic And Electrolytic Cell . there are two different kinds of electrochemical cells: this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. both a galvanic cell and an electrolytic cell have electrical current flowing. Galvanic cells, which have spontaneous redox reactions. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy.
from chem.libretexts.org
there are two different kinds of electrochemical cells: both a galvanic cell and an electrolytic cell have electrical current flowing. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. Galvanic cells, which have spontaneous redox reactions. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the.
Electrolytic Cells Chemistry LibreTexts
Distinguish Between Galvanic And Electrolytic Cell this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. both a galvanic cell and an electrolytic cell have electrical current flowing. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Galvanic cells, which have spontaneous redox reactions. this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. there are two different kinds of electrochemical cells:
From learningschoolletopisaln.z22.web.core.windows.net
Types Of Electrochemical Cells Pdf Distinguish Between Galvanic And Electrolytic Cell both a galvanic cell and an electrolytic cell have electrical current flowing. Galvanic cells, which have spontaneous redox reactions. this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. galvanic cells generate electrical energy. Distinguish Between Galvanic And Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Distinguish Between Galvanic And Electrolytic Cell Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. there are two different kinds of electrochemical cells: Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell is an. Distinguish Between Galvanic And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Distinguish Between Galvanic And Electrolytic Cell Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. both a galvanic cell and an electrolytic cell have electrical current flowing. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. . Distinguish Between Galvanic And Electrolytic Cell.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts Distinguish Between Galvanic And Electrolytic Cell there are two different kinds of electrochemical cells: both a galvanic cell and an electrolytic cell have electrical current flowing. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. this article explains the key differences. Distinguish Between Galvanic And Electrolytic Cell.
From exoyksicy.blob.core.windows.net
Distinguish Between Galvanic Cell And Electrolytic Cell at John Rowe blog Distinguish Between Galvanic And Electrolytic Cell Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. there are two different kinds of electrochemical cells: galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox.. Distinguish Between Galvanic And Electrolytic Cell.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How Distinguish Between Galvanic And Electrolytic Cell there are two different kinds of electrochemical cells: galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Galvanic cells, which have spontaneous redox reactions. both a galvanic cell and an electrolytic cell have electrical current flowing. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing. Distinguish Between Galvanic And Electrolytic Cell.
From jesse-has-lozano.blogspot.com
Explain the Difference Between a Galvanic Cell and Electrolytic Cell Distinguish Between Galvanic And Electrolytic Cell this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. galvanic cells generate electrical energy through spontaneous redox reactions,. Distinguish Between Galvanic And Electrolytic Cell.
From www.pinterest.co.uk
What are the differences between Galvanic cell and Electrolytic cell Distinguish Between Galvanic And Electrolytic Cell there are two different kinds of electrochemical cells: a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. both a galvanic cell and. Distinguish Between Galvanic And Electrolytic Cell.
From exoyksicy.blob.core.windows.net
Distinguish Between Galvanic Cell And Electrolytic Cell at John Rowe blog Distinguish Between Galvanic And Electrolytic Cell galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Galvanic cells utilize a spontaneous flow of electrons. Distinguish Between Galvanic And Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Distinguish Between Galvanic And Electrolytic Cell Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Galvanic cells, which have spontaneous redox reactions. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. both a galvanic cell and an electrolytic cell have electrical current flowing. a galvanic cell is an electrochemical cell in. Distinguish Between Galvanic And Electrolytic Cell.
From hinahanap6dschematic.z21.web.core.windows.net
What Forms At The Cathode During Electrolysis Distinguish Between Galvanic And Electrolytic Cell Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. there are two different kinds of electrochemical cells: galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. both a galvanic cell and. Distinguish Between Galvanic And Electrolytic Cell.
From differguide.com
Difference Between Galvanic And Electrolytic Cell Distinguish Between Galvanic And Electrolytic Cell Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. Galvanic cells, which have spontaneous redox reactions. there are two different kinds of electrochemical cells: both a galvanic cell and an electrolytic cell have electrical current flowing. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. . Distinguish Between Galvanic And Electrolytic Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Distinguish Between Galvanic And Electrolytic Cell this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. there are two different kinds of electrochemical cells: galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an. Distinguish Between Galvanic And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Distinguish Between Galvanic And Electrolytic Cell Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. Galvanic cells, which have spontaneous redox reactions. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. there are two different kinds of electrochemical. Distinguish Between Galvanic And Electrolytic Cell.
From exoyksicy.blob.core.windows.net
Distinguish Between Galvanic Cell And Electrolytic Cell at John Rowe blog Distinguish Between Galvanic And Electrolytic Cell this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. galvanic cells generate electrical energy through spontaneous redox reactions,. Distinguish Between Galvanic And Electrolytic Cell.
From saylordotorg.github.io
Electrolysis Distinguish Between Galvanic And Electrolytic Cell a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. Galvanic cells, which have spontaneous redox reactions. this article explains the key differences. Distinguish Between Galvanic And Electrolytic Cell.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube Distinguish Between Galvanic And Electrolytic Cell Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. both a galvanic cell and an electrolytic cell have electrical current flowing. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. there are two different kinds of electrochemical cells: this article explains the key differences between galvanic. Distinguish Between Galvanic And Electrolytic Cell.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint Distinguish Between Galvanic And Electrolytic Cell this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Galvanic cells utilize a spontaneous flow of electrons to convert. Distinguish Between Galvanic And Electrolytic Cell.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog Distinguish Between Galvanic And Electrolytic Cell a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. both a galvanic cell and an electrolytic cell have electrical current flowing. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. Galvanic cells, which have spontaneous redox reactions. there are two different kinds of electrochemical cells: . Distinguish Between Galvanic And Electrolytic Cell.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Distinguish Between Galvanic And Electrolytic Cell both a galvanic cell and an electrolytic cell have electrical current flowing. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. this article explains the key differences between galvanic cell and electrolytic cell on the basis of. Distinguish Between Galvanic And Electrolytic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Distinguish Between Galvanic And Electrolytic Cell a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert. there are two different kinds of electrochemical cells: Galvanic cells, which have spontaneous redox reactions. this article explains the key differences between galvanic cell and electrolytic cell on. Distinguish Between Galvanic And Electrolytic Cell.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube Distinguish Between Galvanic And Electrolytic Cell this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. there are two different kinds of electrochemical cells: galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Galvanic cells, which have spontaneous redox reactions. both a galvanic cell and an electrolytic. Distinguish Between Galvanic And Electrolytic Cell.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Distinguish Between Galvanic And Electrolytic Cell Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Galvanic cells, which have spontaneous redox reactions. this article explains the key. Distinguish Between Galvanic And Electrolytic Cell.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells Distinguish Between Galvanic And Electrolytic Cell both a galvanic cell and an electrolytic cell have electrical current flowing. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Galvanic cells, which have spontaneous redox reactions. there are two different kinds of electrochemical cells: a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing. Distinguish Between Galvanic And Electrolytic Cell.