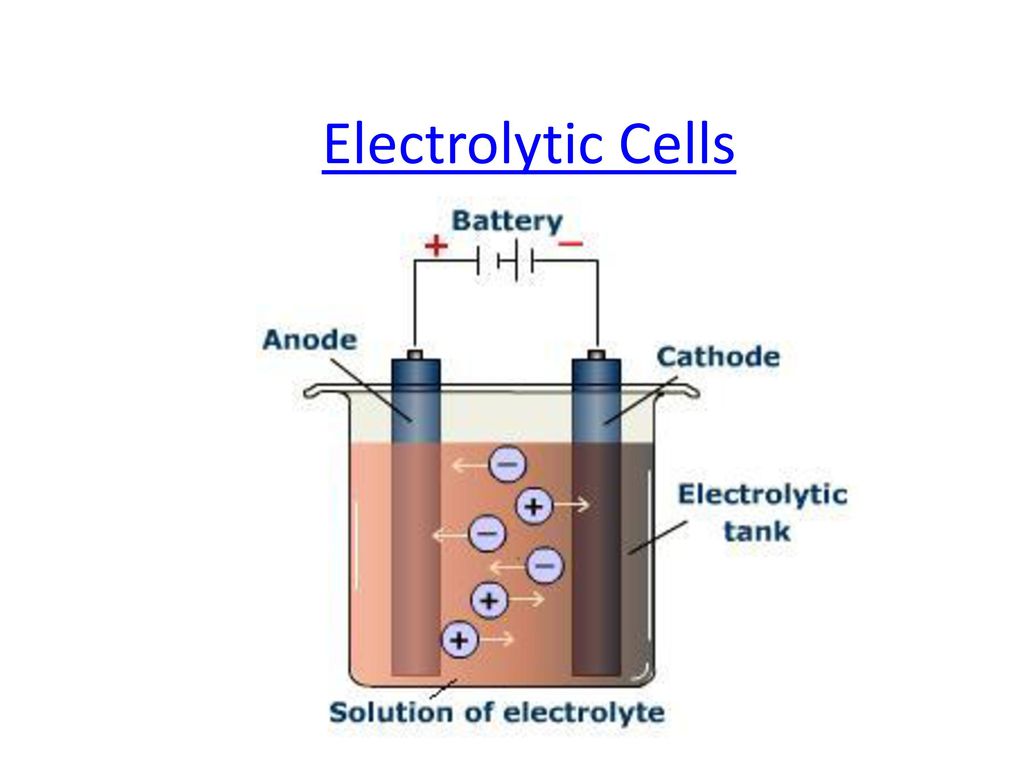
Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell . In a voltaic cell, the oxidation and reduction of metals occurs. an electrode is strip of metal on which the reaction takes place. A number of cells can be connected in series to. A simple cell can be made by connecting two different metals in contact with an electrolyte. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. The arrangement consists of two metals partially submerged in an. A cell, such as in a battery, in which an. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The important parts of a voltaic cell: the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte.
from sciencevision.in
A simple cell can be made by connecting two different metals in contact with an electrolyte. The arrangement consists of two metals partially submerged in an. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A cell, such as in a battery, in which an. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. an electrode is strip of metal on which the reaction takes place. In a voltaic cell, the oxidation and reduction of metals occurs. A number of cells can be connected in series to. The important parts of a voltaic cell:
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision
Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell an electrode is strip of metal on which the reaction takes place. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The arrangement consists of two metals partially submerged in an. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The important parts of a voltaic cell: In a voltaic cell, the oxidation and reduction of metals occurs. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. an electrode is strip of metal on which the reaction takes place. A number of cells can be connected in series to. A simple cell can be made by connecting two different metals in contact with an electrolyte. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. A cell, such as in a battery, in which an.
From guidemanualcodas.z1.web.core.windows.net
Diagram Of Voltaic Cell Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. A cell, such as in a battery, in which an. In a voltaic cell,. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From usermanualtractors.z1.web.core.windows.net
Cathode In Electrochemical Cell Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell A cell, such as in a battery, in which an. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. an electrode is strip of metal on which the reaction takes place. The arrangement consists of two metals. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From hinahanap6dschematic.z21.web.core.windows.net
What Forms At The Cathode During Electrolysis Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell The arrangement consists of two metals partially submerged in an. A number of cells can be connected in series to. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. an electrode is strip of metal on which. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From manualpartwedlock88.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell an electrode is strip of metal on which the reaction takes place. A number of cells can be connected in series to. The important parts of a voltaic cell: the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. the basic cell or voltaic cell is made up of two electrodes, one of copper. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From www.youtube.com
Ch. 203 Inert Electrodes/Voltaic Cells YouTube Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. In a voltaic cell, the oxidation and reduction of metals occurs. A number of cells can be connected in series to. The important parts of a voltaic cell: the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. . Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell A number of cells can be connected in series to. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. A simple cell can be made by connecting two different metals in contact with an electrolyte. an electrode is strip of metal on which the reaction takes place. the basic cell. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From userdiagramkipped.z21.web.core.windows.net
Picture Of Anode Electrolyte Cathode In Cell Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From chemistry.stackexchange.com
electrochemistry Where do negative ions migrate from the salt bridge Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. A number of cells can be connected in series to. an electrode is strip of metal on which the reaction takes place. A simple cell can be made by connecting two different metals in contact with an electrolyte. the simple voltaic. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell an electrode is strip of metal on which the reaction takes place. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. a. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From circuitdbclicheed.z13.web.core.windows.net
Electrochemical Cell Diagram Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. In a voltaic cell, the oxidation and reduction of metals occurs. the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. A cell, such. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell In a voltaic cell, the oxidation and reduction of metals occurs. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. a galvanic. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 11 simple voltaic cell Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. The important parts of a voltaic cell: A number of cells can be connected in series to. the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. an electrode is strip of metal on which the reaction takes place. . Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell The arrangement consists of two metals partially submerged in an. A cell, such as in a battery, in which an. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. the simple voltaic (or galvanic) electrical cell was. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell an electrode is strip of metal on which the reaction takes place. A number of cells can be connected in series to. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. a voltaic cell is. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From schematiclistpact101.z22.web.core.windows.net
Chemical Cell Diagram Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell The important parts of a voltaic cell: an electrode is strip of metal on which the reaction takes place. A number of cells can be connected in series to. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A simple cell can be made by connecting two different metals in contact. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell A simple cell can be made by connecting two different metals in contact with an electrolyte. the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. A number of cells can be connected in series to. In a voltaic cell, the oxidation and reduction of metals occurs. The arrangement consists of two metals partially submerged in. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From keyesvannuyshyundai.blogspot.com
draw the diagram of electrolytic cell and explain keyesvannuyshyundai Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell The arrangement consists of two metals partially submerged in an. The important parts of a voltaic cell: In a voltaic cell, the oxidation and reduction of metals occurs. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. A. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell In a voltaic cell, the oxidation and reduction of metals occurs. A number of cells can be connected in series to. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. A cell, such as in a battery, in which an. The important parts of a voltaic cell: the simple voltaic (or. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From circuitlibrarybauer.z13.web.core.windows.net
Strong Electrolyte Diagram Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell A number of cells can be connected in series to. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The important parts of a voltaic cell: The arrangement consists of two metals partially submerged in an. A simple cell can be made by connecting two different metals in contact with an electrolyte.. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From guidediagrampreconsume.z21.web.core.windows.net
Simple Cell Diagram Chemistry Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. The important parts of a voltaic cell: In a voltaic cell, the oxidation and reduction of metals occurs. the basic cell or voltaic cell is made up. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From wiringdbmamadoup4v.z22.web.core.windows.net
Draw A Labelled Diagram Of Electrolytic Cell Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. A cell, such as in a battery, in which an. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. the simple voltaic. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. A number of cells can be connected in series to. A simple cell can be made by connecting two different metals in contact with an electrolyte. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell The important parts of a voltaic cell: The arrangement consists of two metals partially submerged in an. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. A simple cell can be made by connecting two different metals. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From circuitlibrarylinty.z13.web.core.windows.net
Voltaic Cell Diagram Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell A cell, such as in a battery, in which an. A simple cell can be made by connecting two different metals in contact with an electrolyte. The arrangement consists of two metals partially submerged in an. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. an electrode is strip of metal on which. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The arrangement consists of two metals partially submerged in an. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. A cell, such as in a battery, in which an. an electrode is strip of. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell The important parts of a voltaic cell: an electrode is strip of metal on which the reaction takes place. In a voltaic cell, the oxidation and reduction of metals occurs. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. a galvanic (voltaic) cell converts the energy released by a spontaneous. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From quizdbpharmacies.z4.web.core.windows.net
How Do Electrons Flow In A Voltaic Cell Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell A number of cells can be connected in series to. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. In a. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell The important parts of a voltaic cell: the basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. A simple cell can be made by connecting two different metals in contact with an electrolyte. Either of the two parts of. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The arrangement consists of two metals partially submerged in an. A cell, such as in a battery, in which an. A number of cells can be connected in series to. The important parts of a voltaic cell: the simple voltaic (or galvanic). Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From chemwiki.ucdavis.edu
Voltaic Cells Chemwiki Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell The important parts of a voltaic cell: an electrode is strip of metal on which the reaction takes place. a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. In a voltaic cell, the oxidation and reduction of metals occurs. A simple cell can be made by connecting two different metals in. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell A cell, such as in a battery, in which an. a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A simple cell can be made by connecting two different metals in contact with an electrolyte. In a voltaic cell, the oxidation and reduction of metals occurs. Either of the two parts of. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From saylordotorg.github.io
Electrochemistry Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. The important parts of a voltaic cell: the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. A cell, such as in a battery, in which an. A number of cells can be connected in series to. The arrangement consists of. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell a voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. In a voltaic cell, the oxidation and reduction of metals occurs. The important parts of a voltaic cell: the simple voltaic (or galvanic) electrical cell was developed in the early 1800s. A simple cell can be made by connecting two different metals. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.
From circuitlistjames.z21.web.core.windows.net
Diagram Of A Simple Electrolytic Cell Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell A simple cell can be made by connecting two different metals in contact with an electrolyte. Either of the two parts of an electrochemical cell containing an electrode and an electrolyte. The important parts of a voltaic cell: a galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. a voltaic cell is. Which Electrode And Electrolyte Is Used In A Simple Voltaic Cell.