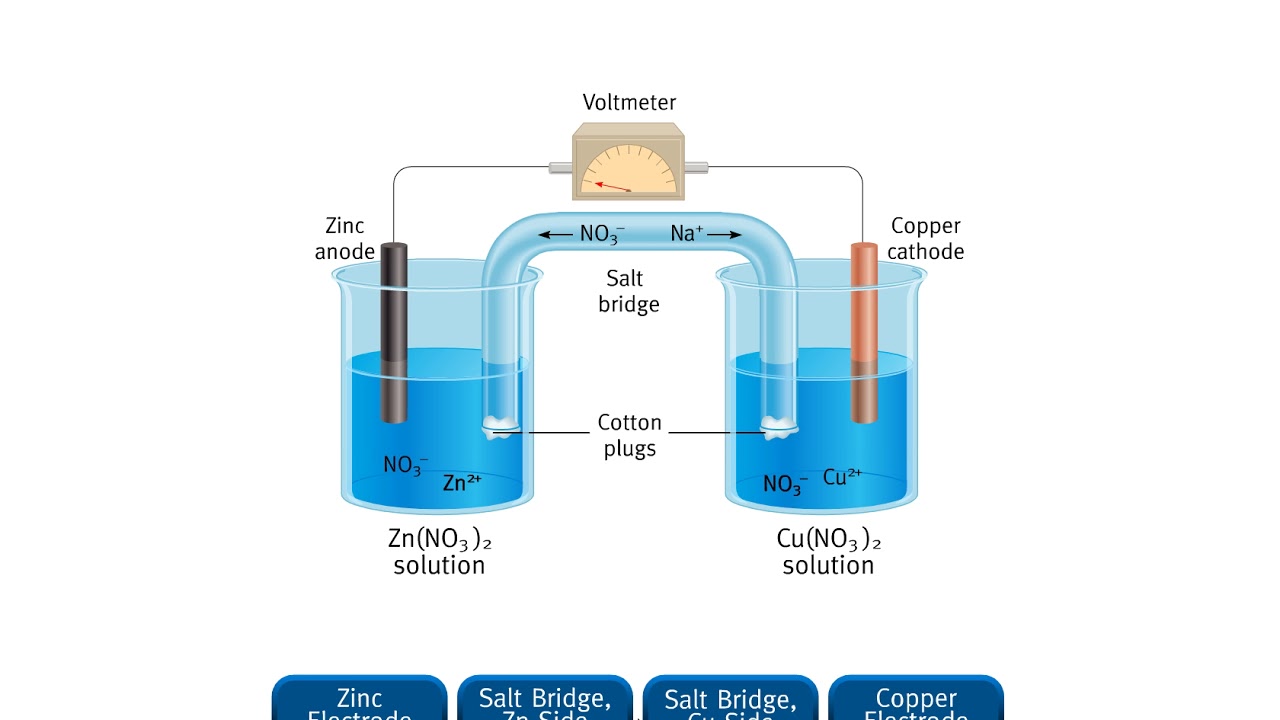
Zn Cu Electrode Potential . for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. 5.6.1 define standard electrode potential, e°, and. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. #standardelectrodepotential #electrochemistrywe know we measure the. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. There is an equilibrium between t. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the.
from www.youtube.com
for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. #standardelectrodepotential #electrochemistrywe know we measure the. There is an equilibrium between t. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. 5.6.1 define standard electrode potential, e°, and. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework.
Electrochemical cell Zn and Cu YouTube
Zn Cu Electrode Potential This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. 5.6.1 define standard electrode potential, e°, and. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. #standardelectrodepotential #electrochemistrywe know we measure the. There is an equilibrium between t. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v.
From www.youtube.com
Explain the origin of single electrode potential? Electrochemistry Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. #standardelectrodepotential #electrochemistrywe know we measure the. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10. Zn Cu Electrode Potential.
From 2012books.lardbucket.org
Electrochemistry Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas. Zn Cu Electrode Potential.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Zn Cu Electrode Potential in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. . Zn Cu Electrode Potential.
From fixwiringviridite.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Zn Cu Electrode Potential for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. tabulating all electrode potentials with respect to the same standard electrode provides a. Zn Cu Electrode Potential.
From saylordotorg.github.io
Standard Potentials Zn Cu Electrode Potential 5.6.1 define standard electrode potential, e°, and. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is. Zn Cu Electrode Potential.
From monomole.com
Standard electrode potentials Mono Mole Zn Cu Electrode Potential for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. There is an equilibrium between t. #standardelectrodepotential #electrochemistrywe know we measure the. . Zn Cu Electrode Potential.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Zn Cu Electrode Potential 5.6.1 define standard electrode potential, e°, and. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq). Zn Cu Electrode Potential.
From kunduz.com
[ANSWERED] The standard electrode potential of Zn Ag and Cu electrodes Zn Cu Electrode Potential 5.6.1 define standard electrode potential, e°, and. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. #standardelectrodepotential #electrochemistrywe know we measure the. for example,. Zn Cu Electrode Potential.
From www.xiaokudang.com
《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)10 Introduction to Zn Cu Electrode Potential This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. 5.6.1 define standard electrode potential, e°, and. tabulating all electrode potentials with. Zn Cu Electrode Potential.
From app.pandai.org
Determine oxidising agent and reducing agent based on value of standard Zn Cu Electrode Potential There is an equilibrium between t. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. 5.6.1 define standard electrode potential, e°, and.. Zn Cu Electrode Potential.
From www.chegg.com
Solved 1. Predict what will happen to the Zn electrode and Zn Cu Electrode Potential There is an equilibrium between t. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. This implies that the potential difference between the co and cu. Zn Cu Electrode Potential.
From www.xiaokudang.com
《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)10 Introduction to Zn Cu Electrode Potential This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. #standardelectrodepotential #electrochemistrywe know we measure the. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−}. Zn Cu Electrode Potential.
From hinahanap6dschematic.z21.web.core.windows.net
What Forms At The Cathode During Electrolysis Zn Cu Electrode Potential in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. tabulating all electrode potentials with respect to. Zn Cu Electrode Potential.
From zhuanlan.zhihu.com
南通大学Adv. Mater.:原位Raman用于可视化锌沉积过程中电极/电解质界面处锌离子的变化 知乎 Zn Cu Electrode Potential There is an equilibrium between t. #standardelectrodepotential #electrochemistrywe know we measure the. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Zn Cu Electrode Potential.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Zn Cu Electrode Potential for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. for example, the measured standard cell potential. Zn Cu Electrode Potential.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. There is an equilibrium between t. #standardelectrodepotential #electrochemistrywe know we measure the. This implies that the potential difference between the co. Zn Cu Electrode Potential.
From www.coursehero.com
[Solved] Lab 14 Electrochemical Cells How does your measured electrode Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because. Zn Cu Electrode Potential.
From ncerthelp.blogspot.com
NCERT Solutions, CBSE Sample Papers and Syllabus for Class 9 to 12 Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. There is an equilibrium between t. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) +. Zn Cu Electrode Potential.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. There is an equilibrium between t. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) +. Zn Cu Electrode Potential.
From monomole.com
Standard electrode potentials Mono Mole Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper. Zn Cu Electrode Potential.
From 2012books.lardbucket.org
Electrochemistry Zn Cu Electrode Potential for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. There is an equilibrium between t. #standardelectrodepotential #electrochemistrywe know we measure the. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. . Zn Cu Electrode Potential.
From www.chegg.com
Solved Standard Electrode Potentials in Aqueous Solution at Zn Cu Electrode Potential 5.6.1 define standard electrode potential, e°, and. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. tabulating all electrode potentials with respect. Zn Cu Electrode Potential.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Atoms First Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. 5.6.1 define standard electrode potential, e°, and. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10. Zn Cu Electrode Potential.
From exoxyzops.blob.core.windows.net
One Example Of Electrochemical Cell at Kristen Martin blog Zn Cu Electrode Potential There is an equilibrium between t. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. in the zn/cu system, the valence electrons in zinc have. Zn Cu Electrode Potential.
From www.toppr.com
The cell reaction in Daniel cell is Zn(s) + Cu^2 + (aq)→ Zn^2 + (aq Zn Cu Electrode Potential This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} →. Zn Cu Electrode Potential.
From monomole.com
Standard electrode potentials Mono Mole Zn Cu Electrode Potential 5.6.1 define standard electrode potential, e°, and. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the corresponding zn/co system is 0.51 v. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. #standardelectrodepotential #electrochemistrywe know we measure. Zn Cu Electrode Potential.
From www.vedclass.com
If the standard reduction potential for four divalent elements X,, Y Zn Cu Electrode Potential in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. #standardelectrodepotential #electrochemistrywe know we measure the. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10. Zn Cu Electrode Potential.
From www.youtube.com
Determination of Standard Electrode potential of 'Zn and Cu Zn Cu Electrode Potential 5.6.1 define standard electrode potential, e°, and. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. This implies that the potential difference between the co and. Zn Cu Electrode Potential.
From monomole.com
Standard electrode potentials Mono Mole Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. There is an equilibrium between t. #standardelectrodepotential #electrochemistrywe know we measure the. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. 5.6.1 define standard electrode potential, e°, and. in. Zn Cu Electrode Potential.
From kunduz.com
[ANSWERED] The standard electrode potential of Zn Ag and Cu are 0 76 0 Zn Cu Electrode Potential #standardelectrodepotential #electrochemistrywe know we measure the. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. in the zn/cu system, the valence electrons in zinc have a substantially higher potential. Zn Cu Electrode Potential.
From byjus.com
Variation of Cell Potential in ZnCu Cell Chemistry Practicals Class Zn Cu Electrode Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. 5.6.1 define standard electrode potential, e°, and. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v,. Zn Cu Electrode Potential.
From askfilo.com
The graph of electrode potential of Cu half cell ECu−2/Cu vs log[Cu2+] i.. Zn Cu Electrode Potential for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence. Zn Cu Electrode Potential.
From saylordotorg.github.io
Standard Potentials Zn Cu Electrode Potential This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. in this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. . Zn Cu Electrode Potential.
From byjus.com
the s†an dard electrode potential of Zn ,Ag and Cu are 0.76 ,0.80 and 0 Zn Cu Electrode Potential There is an equilibrium between t. This implies that the potential difference between the co and cu electrodes is 1.10 v − 0.51 v = 0.59 v. 5.6.1 define standard electrode potential, e°, and. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of.. Zn Cu Electrode Potential.
From www.researchgate.net
Zn growth schematics of bare Zn and 3DCucoated electrodes. (A,B) Bare Zn Cu Electrode Potential There is an equilibrium between t. 5.6.1 define standard electrode potential, e°, and. #standardelectrodepotential #electrochemistrywe know we measure the. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. for example, the measured standard cell potential (e°) for the zn/cu system is 1.10 v, whereas e° for the. in this. Zn Cu Electrode Potential.