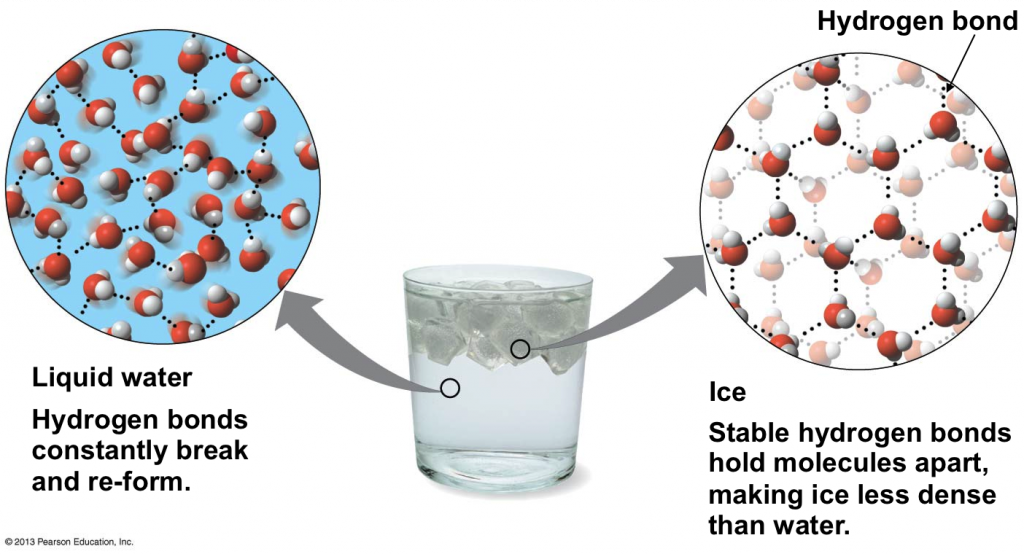
Does One Water Molecule Have The Same Density As A Drop Of Water . Students measure the volume and mass of water to determine its density. Students measure the volume and mass of water to determine its density. Then they measure the mass of. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. One mole of water is 18.016 grams, so in. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. The bonds between water molecules are called hydrogen bonds. Then they measure the mass of different volumes of. As water cools to 3.98°c, its mass stays.
from punchlistzero.com
Then they measure the mass of different volumes of. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. As water cools to 3.98°c, its mass stays. The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. One mole of water is 18.016 grams, so in. The bonds between water molecules are called hydrogen bonds. Then they measure the mass of. Students measure the volume and mass of water to determine its density.
Specific Heat of Ice In Various Units, vs. Water,
Does One Water Molecule Have The Same Density As A Drop Of Water Students measure the volume and mass of water to determine its density. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Students measure the volume and mass of water to determine its density. The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. Then they measure the mass of. As water cools to 3.98°c, its mass stays. One mole of water is 18.016 grams, so in. The bonds between water molecules are called hydrogen bonds. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. Students measure the volume and mass of water to determine its density. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. Then they measure the mass of different volumes of. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom.
From www.futureengineers.org
Future Engineers Name that Molecule Challenge Gallery Water Does One Water Molecule Have The Same Density As A Drop Of Water Students measure the volume and mass of water to determine its density. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. As water cools to 3.98°c, its mass stays. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. The molecules in a single drop of water. Does One Water Molecule Have The Same Density As A Drop Of Water.
From student-tutor.com
Understanding the Density of Water StudentTutor Education Blog Does One Water Molecule Have The Same Density As A Drop Of Water As water cools to 3.98°c, its mass stays. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. Properties of water include its chemical formula h2o, density, melting, boiling. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.pinterest.com
Why Is Water a Polar Molecule? Water molecule, Molecular geometry Does One Water Molecule Have The Same Density As A Drop Of Water Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. As water cools to 3.98°c, its mass stays. The bonds between water molecules are called hydrogen bonds. The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. One. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. Does One Water Molecule Have The Same Density As A Drop Of Water As water cools to 3.98°c, its mass stays. Then they measure the mass of different volumes of. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. One mole of water is 18.016 grams, so in. The bonds between water molecules are called hydrogen bonds. Students measure the volume and mass of water. Does One Water Molecule Have The Same Density As A Drop Of Water.
From byjus.com
In water, oxygen has a slight negative charge and hydrogen has a slight Does One Water Molecule Have The Same Density As A Drop Of Water As water cools to 3.98°c, its mass stays. Students measure the volume and mass of water to determine its density. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Then they measure the mass of different volumes of. Water is a polar molecule, as greater electron density is found around the more electronegative. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.pngkey.com
Hydrogen Bonding Is The Effect Of Water Molecules Attracted 5 Does One Water Molecule Have The Same Density As A Drop Of Water As water cools to 3.98°c, its mass stays. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Students measure the volume and mass of. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.ces.fau.edu
Climate Science Investigations South Florida Temperature Over Time Does One Water Molecule Have The Same Density As A Drop Of Water Students measure the volume and mass of water to determine its density. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. Then they measure. Does One Water Molecule Have The Same Density As A Drop Of Water.
From dokumen.tips
(PPTX) Solutions, Acids & Bases. The Water Molecule How many protons Does One Water Molecule Have The Same Density As A Drop Of Water Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Then they measure the mass of. Students measure the volume and mass of water to determine its density. The bonds between water molecules are called hydrogen bonds. A drop of water. Does One Water Molecule Have The Same Density As A Drop Of Water.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure Does One Water Molecule Have The Same Density As A Drop Of Water Students measure the volume and mass of water to determine its density. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. The molecules in. Does One Water Molecule Have The Same Density As A Drop Of Water.
From zlaj-vredina.blogspot.com
Best Of How Is Water Molecule Like A Does One Water Molecule Have The Same Density As A Drop Of Water As water cools to 3.98°c, its mass stays. The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. Then they measure the mass of different volumes of. The bonds between water molecules are called hydrogen bonds. One mole of water is 18.016 grams, so in.. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.youtube.com
Number of Molecules in One Liter of Water YouTube Does One Water Molecule Have The Same Density As A Drop Of Water Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o). Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.acs.org
Lesson 5.1 Water is a Polar Molecule American Chemical Society Does One Water Molecule Have The Same Density As A Drop Of Water Then they measure the mass of different volumes of. Students measure the volume and mass of water to determine its density. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Each water molecule is made up of two hydrogen (h). Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.universallifetools.com
Liquid Crystalline Body Structured Water Does One Water Molecule Have The Same Density As A Drop Of Water Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Students measure the volume and mass of water to determine its density. The bonds between water molecules are called hydrogen bonds. Then they measure the mass of. Does One Water Molecule Have The Same Density As A Drop Of Water.
From worldoceanreview.com
Water a unique molecule « World Ocean Review Does One Water Molecule Have The Same Density As A Drop Of Water As water cools to 3.98°c, its mass stays. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Students measure the volume and mass of water to determine its density. One mole of water is 18.016 grams, so in. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o). Does One Water Molecule Have The Same Density As A Drop Of Water.
From wt.kimiq.com
The Shape Of The Water Molecule H2o Is Water Ionizer Does One Water Molecule Have The Same Density As A Drop Of Water A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. As water cools to 3.98°c, its mass stays. Students measure the volume and mass of water to determine its density. Water is a polar molecule, as greater electron. Does One Water Molecule Have The Same Density As A Drop Of Water.
From alevelbiology.co.uk
Dipoles Of Water Molecules ALevel Biology Revision Notes Does One Water Molecule Have The Same Density As A Drop Of Water Students measure the volume and mass of water to determine its density. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Then they measure the mass of different volumes of. As water cools to 3.98°c, its mass stays. Students measure the volume and mass of water to determine its density. Each water molecule. Does One Water Molecule Have The Same Density As A Drop Of Water.
From pandai.me
Objects or Materials which are More or Less Dense than Water Does One Water Molecule Have The Same Density As A Drop Of Water The bonds between water molecules are called hydrogen bonds. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Students measure the volume and mass of water to determine its density. Properties of water include its chemical. Does One Water Molecule Have The Same Density As A Drop Of Water.
From ar.inspiredpencil.com
Density Examples Of Water Does One Water Molecule Have The Same Density As A Drop Of Water Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. As water cools to 3.98°c, its mass stays. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. One mole of water is 18.016 grams, so in. Properties of water include its chemical formula h2o, density, melting,. Does One Water Molecule Have The Same Density As A Drop Of Water.
From byjus.com
Why is water considered a compound? Does One Water Molecule Have The Same Density As A Drop Of Water Students measure the volume and mass of water to determine its density. Students measure the volume and mass of water to determine its density. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.thermal-engineering.org
What is Density Physics Definition Does One Water Molecule Have The Same Density As A Drop Of Water Then they measure the mass of different volumes of. The bonds between water molecules are called hydrogen bonds. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. As water cools to 3.98°c, its mass stays. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. Students. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.britannica.com
Water Definition, Chemical Formula, Structure, Molecule, & Facts Does One Water Molecule Have The Same Density As A Drop Of Water Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. The bonds between water molecules are called hydrogen bonds. Then they measure the mass of different volumes of. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a. Does One Water Molecule Have The Same Density As A Drop Of Water.
From owlcation.com
Primary and Secondary Bonds Owlcation Does One Water Molecule Have The Same Density As A Drop Of Water The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. Then they measure the mass of. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Properties of water include its chemical formula h2o, density, melting, boiling point &. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.medicalsciencenavigator.com
Physiology of Cell Signaling Does One Water Molecule Have The Same Density As A Drop Of Water One mole of water is 18.016 grams, so in. The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. Then they measure the mass of. Students measure the volume and mass of water to determine its density. As water cools to 3.98°c, its mass stays.. Does One Water Molecule Have The Same Density As A Drop Of Water.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Does One Water Molecule Have The Same Density As A Drop Of Water Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. The bonds between water molecules are called hydrogen bonds. As water cools to 3.98°c, its mass stays. Students measure the volume and mass of water to determine its density. Students measure the volume and mass of water to determine its density. The molecules in. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.expii.com
Density of Water — Comparison of Solid vs. Liquid Expii Does One Water Molecule Have The Same Density As A Drop Of Water Students measure the volume and mass of water to determine its density. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. Students measure the volume and mass of water to determine its density. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. Then they measure. Does One Water Molecule Have The Same Density As A Drop Of Water.
From sciencetrends.com
Polar Molecule Definition And Examples Science Trends Does One Water Molecule Have The Same Density As A Drop Of Water Then they measure the mass of. The bonds between water molecules are called hydrogen bonds. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. The molecules in a single drop of water diluted evenly in the earth’s oceans would result. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.scienceabc.com
Is Water Polar Or Nonpolar? » ScienceABC Does One Water Molecule Have The Same Density As A Drop Of Water The molecules in a single drop of water diluted evenly in the earth’s oceans would result in the density of one molecule per litre of. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. One mole of water is 18.016. Does One Water Molecule Have The Same Density As A Drop Of Water.
From courses.lumenlearning.com
10.1 Intermolecular Forces Chemistry Does One Water Molecule Have The Same Density As A Drop Of Water One mole of water is 18.016 grams, so in. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. A drop of water is. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.pinterest.co.kr
Water Expansion When Freezing Science Facts Water molecule Does One Water Molecule Have The Same Density As A Drop Of Water One mole of water is 18.016 grams, so in. Then they measure the mass of different volumes of. Students measure the volume and mass of water to determine its density. The bonds between water molecules are called hydrogen bonds. As water cools to 3.98°c, its mass stays. Then they measure the mass of. The molecules in a single drop of. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.acs.org
Lesson 5.1 Water is a Polar Molecule American Chemical Society Does One Water Molecule Have The Same Density As A Drop Of Water The bonds between water molecules are called hydrogen bonds. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Students measure the volume and mass of water to determine its density. Then they measure the mass of. A drop of water. Does One Water Molecule Have The Same Density As A Drop Of Water.
From gotbooks.miracosta.edu
gotbooks.miracosta.edu/oceans Does One Water Molecule Have The Same Density As A Drop Of Water Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. One mole of water is 18.016 grams, so in. Students measure the volume and mass of water to determine its density. The bonds between water molecules are called hydrogen bonds. Water is a polar molecule, as greater electron density is found around the more. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.expii.com
High Specific Heat (Water) — Properties & Examples Expii Does One Water Molecule Have The Same Density As A Drop Of Water Then they measure the mass of different volumes of. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Then they measure the mass of. The bonds between water molecules are called hydrogen bonds. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two. Does One Water Molecule Have The Same Density As A Drop Of Water.
From biostudizz.weebly.com
WATER CHEMISTRY to Bio Stud... Does One Water Molecule Have The Same Density As A Drop Of Water As water cools to 3.98°c, its mass stays. The bonds between water molecules are called hydrogen bonds. Then they measure the mass of different volumes of. Students measure the volume and mass of water to determine its density. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. Properties of water include its chemical. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.expii.com
Water — Molecular Structure & Bonding Expii Does One Water Molecule Have The Same Density As A Drop Of Water Then they measure the mass of. Each water molecule is made up of two hydrogen (h) atoms and one oxygen (o) atom. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. One mole of water is. Does One Water Molecule Have The Same Density As A Drop Of Water.
From www.vecteezy.com
H2o mollecule Properties and Chemical Compound Structure water consist Does One Water Molecule Have The Same Density As A Drop Of Water Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. A drop of water is 0.05 ml of water, so its mass would be 0.05 grams. As water cools to 3.98°c, its mass stays. The molecules in a single drop of. Does One Water Molecule Have The Same Density As A Drop Of Water.