Electrode Reaction Meaning . An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. When the current leaves the electrode it. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. This movement of electrons is called electricity, which can be generated by movements of. Electrodes are vital components of electrochemical cells. This reaction may take place in a. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. The electrode definition is as follows: Electrochemistry is the study of chemical processes that cause electrons to move.
from www.youtube.com
An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. Electrodes are vital components of electrochemical cells. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. This movement of electrons is called electricity, which can be generated by movements of. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. Electrochemistry is the study of chemical processes that cause electrons to move. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. The electrode definition is as follows: When the current leaves the electrode it.
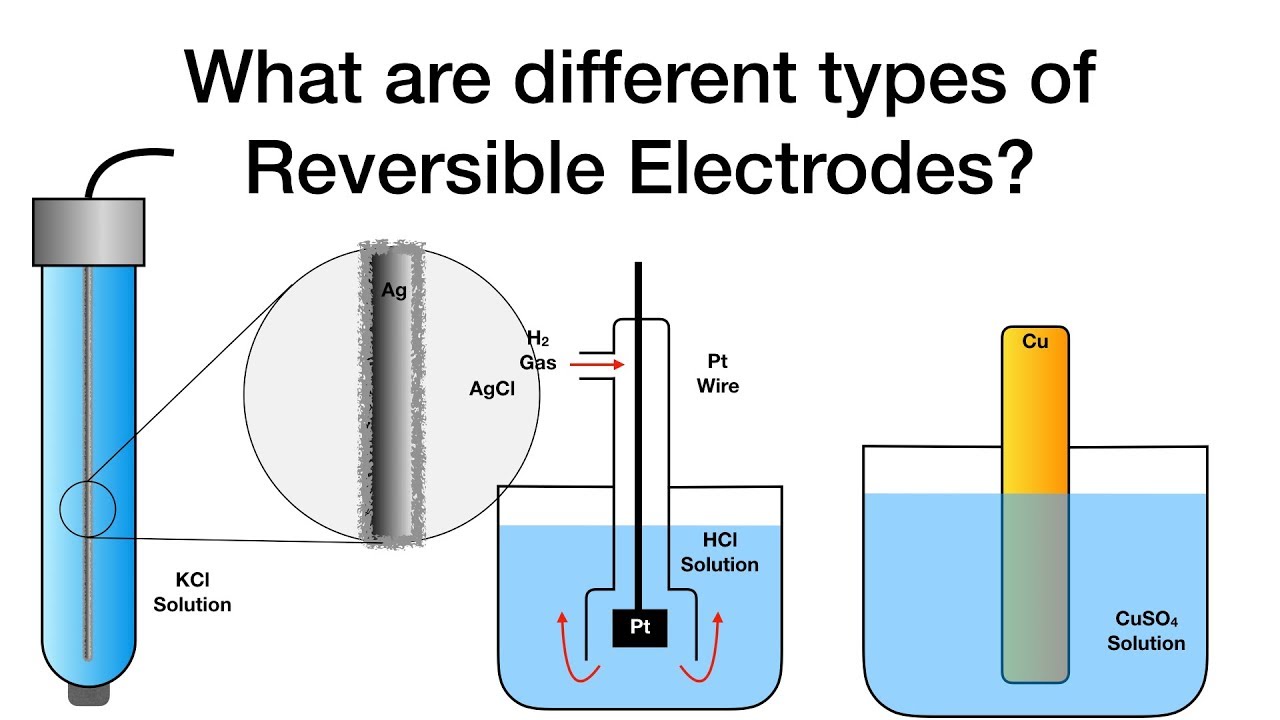
What are different types of Reversible Electrodes? Electrochemistry
Electrode Reaction Meaning This movement of electrons is called electricity, which can be generated by movements of. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. Electrodes are vital components of electrochemical cells. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. This movement of electrons is called electricity, which can be generated by movements of. This reaction may take place in a. The electrode definition is as follows: When the current leaves the electrode it. Electrochemistry is the study of chemical processes that cause electrons to move.
From www.chemistry-teaching-resources.com
chemistry picture Electrode Reaction Meaning When the current leaves the electrode it. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. This movement of electrons is called electricity, which can be generated by movements of. Electrodes are vital components of electrochemical cells. Electrode reactions are a class of chemical reactions that involve the transfer of a charged. Electrode Reaction Meaning.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Electrode Reaction Meaning The electrode definition is as follows: Electrochemistry is the study of chemical processes that cause electrons to move. Electrodes are vital components of electrochemical cells. This reaction may take place in a. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. When the current leaves the electrode it. An. Electrode Reaction Meaning.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Electrode Reaction Meaning The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. This reaction may take place in a. Electrodes are vital components of electrochemical cells. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. Electrode reactions are heterogeneous chemical processes that. Electrode Reaction Meaning.
From www.goodscience.com.au
Extraction of Metals Good Science Electrode Reaction Meaning An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. Electrochemistry is the study of chemical processes that cause electrons to move. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reactions are heterogeneous chemical processes that involve one or. Electrode Reaction Meaning.
From www.youtube.com
What are different types of Reversible Electrodes? Electrochemistry Electrode Reaction Meaning An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. Electrochemistry is the study of chemical processes that cause electrons to move. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reactions are heterogeneous chemical processes that involve one or. Electrode Reaction Meaning.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? WorldAtlas Electrode Reaction Meaning Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. This movement of electrons is called electricity, which can be generated by movements of. This reaction may take place in a. An. Electrode Reaction Meaning.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrode Reaction Meaning This reaction may take place in a. This movement of electrons is called electricity, which can be generated by movements of. The electrode definition is as follows: When the current leaves the electrode it. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. An electrode reaction refers to the net oxidation or reduction. Electrode Reaction Meaning.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Electrode Reaction Meaning This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry is the study of chemical processes that cause electrons to move. The electrode definition is as follows: An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. This reaction may take place in a. An electrode can be. Electrode Reaction Meaning.
From socratic.org
The following cell is operated as an electrolytic cell, using a current Electrode Reaction Meaning Electrodes are vital components of electrochemical cells. The electrode definition is as follows: Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. An electrode can be defined as the point where current either. Electrode Reaction Meaning.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Electrode Reaction Meaning When the current leaves the electrode it. Electrodes are vital components of electrochemical cells. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. An electrode reaction refers to the net oxidation or reduction. Electrode Reaction Meaning.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrode Reaction Meaning An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. Electrochemistry is the study of chemical processes that cause electrons to move. Electrodes are vital components of electrochemical cells. This reaction may take place in a.. Electrode Reaction Meaning.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts Electrode Reaction Meaning Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. Electrochemistry is the study of chemical processes that cause electrons to move. The electrode is an electrical conductor that carries electric current. Electrode Reaction Meaning.
From users.highland.edu
Standard Potentials Electrode Reaction Meaning Electrodes are vital components of electrochemical cells. This movement of electrons is called electricity, which can be generated by movements of. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. The electrode definition is as follows: Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of. Electrode Reaction Meaning.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Reaction Meaning An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrochemistry is the study of chemical processes that cause electrons to move. Electrodes are vital components of electrochemical cells. Electrode reactions are. Electrode Reaction Meaning.
From chembook.org
Standard Potential Electrode Reaction Meaning Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. The electrode definition is as follows: This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry is the study of chemical processes that cause electrons to move. When the current leaves the electrode it. Electrode. Electrode Reaction Meaning.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using Electrode Reaction Meaning This reaction may take place in a. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. When the current leaves the electrode it. Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of.. Electrode Reaction Meaning.
From chem.libretexts.org
6.2 Standard Electrode Potentials Chemistry LibreTexts Electrode Reaction Meaning When the current leaves the electrode it. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of. Electrode reactions are a class of chemical reactions that involve. Electrode Reaction Meaning.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Reaction Meaning The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. This reaction may take place in a. When the current leaves the electrode it. This movement of electrons is called electricity,. Electrode Reaction Meaning.
From www.researchgate.net
Pathway of a general electrode reaction. Download Scientific Diagram Electrode Reaction Meaning The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. This movement of electrons is called electricity, which can be generated by movements of. Electrodes are vital components of. Electrode Reaction Meaning.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Electrode Reaction Meaning An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. This movement of electrons is called electricity, which can be generated by movements of. This reaction may take place in a. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an.. Electrode Reaction Meaning.
From www.youtube.com
Electrochem Eng L0308 Polarization curve and example for an electrode Electrode Reaction Meaning Electrodes are vital components of electrochemical cells. The electrode definition is as follows: Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. An electrode reaction refers to the net oxidation or reduction process. Electrode Reaction Meaning.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Electrode Reaction Meaning This reaction may take place in a. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. The electrode definition is as follows: Electrochemistry is the study of chemical processes that cause electrons to move.. Electrode Reaction Meaning.
From pubs.acs.org
Accurate Potentials of Hg/HgO Electrodes Practical Parameters for Electrode Reaction Meaning Electrochemistry is the study of chemical processes that cause electrons to move. This reaction may take place in a. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. The electrode definition is as follows: Electrodes are vital components of electrochemical cells. This movement of electrons is called electricity,. Electrode Reaction Meaning.
From www.slideserve.com
PPT Chapter 3 of Electrode Reactions PowerPoint Presentation Electrode Reaction Meaning When the current leaves the electrode it. Electrodes are vital components of electrochemical cells. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. This reaction may take place in a. This. Electrode Reaction Meaning.
From webmis.highland.cc.il.us
Electrolysis Electrode Reaction Meaning When the current leaves the electrode it. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. Electrode Reaction Meaning.
From slidetodoc.com
Types of Electrodes and Electrode Reactions An electrode Electrode Reaction Meaning Electrochemistry is the study of chemical processes that cause electrons to move. Electrodes are vital components of electrochemical cells. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. The electrode definition is as follows: This movement of electrons is called electricity, which can be generated by movements of. Electrode reactions are a class. Electrode Reaction Meaning.
From www.nagwa.com
Question Video Identifying Conditions for Measuring Standard Electrode Electrode Reaction Meaning Electrochemistry is the study of chemical processes that cause electrons to move. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. When the current leaves the electrode it. This reaction may take place in a. Electrodes are vital components of electrochemical cells. An electrode can be defined as. Electrode Reaction Meaning.
From saylordotorg.github.io
Electrochemistry Electrode Reaction Meaning An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. This movement of electrons is called electricity, which can be generated by movements of. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. The electrode is an electrical conductor that carries electric current to. Electrode Reaction Meaning.
From www.collegesearch.in
Cathode and Anode Definition, Examples, Differences CollegeSearch Electrode Reaction Meaning Electrochemistry is the study of chemical processes that cause electrons to move. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. The electrode definition is as follows: An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. When the current leaves the electrode it. Electrode. Electrode Reaction Meaning.
From blog.thepipingmart.com
Why are carbon electrodes used in electrolysis? Electrode Reaction Meaning Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an. When the current leaves the electrode it. Electrodes are vital components of electrochemical cells. This reaction may take place in a. This movement. Electrode Reaction Meaning.
From www.researchgate.net
Schematic diagram of the electrode reactions for the electrodes with Electrode Reaction Meaning Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. This reaction may take place in a. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrode reactions. Electrode Reaction Meaning.
From exohmihef.blob.core.windows.net
Standard Electrode Potential Meaning at Francis McQuay blog Electrode Reaction Meaning This reaction may take place in a. The electrode definition is as follows: This movement of electrons is called electricity, which can be generated by movements of. Electrodes are vital components of electrochemical cells. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. When the current leaves the electrode it. Electrode reactions. Electrode Reaction Meaning.
From keypoint.ng
Electrodes Meaning — Chemistry Keypoint Electrode Reaction Meaning Electrodes are vital components of electrochemical cells. This reaction may take place in a. When the current leaves the electrode it. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. Electrochemistry is the study of chemical. Electrode Reaction Meaning.
From www.thoughtco.com
How to Define Anode and Cathode Electrode Reaction Meaning The electrode definition is as follows: Electrode reactions are heterogeneous chemical processes that involve one or more steps with transfer of charge. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit, such as an.. Electrode Reaction Meaning.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Electrode Reaction Meaning An electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. When the current leaves the electrode it. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of a circuit,. Electrode Reaction Meaning.