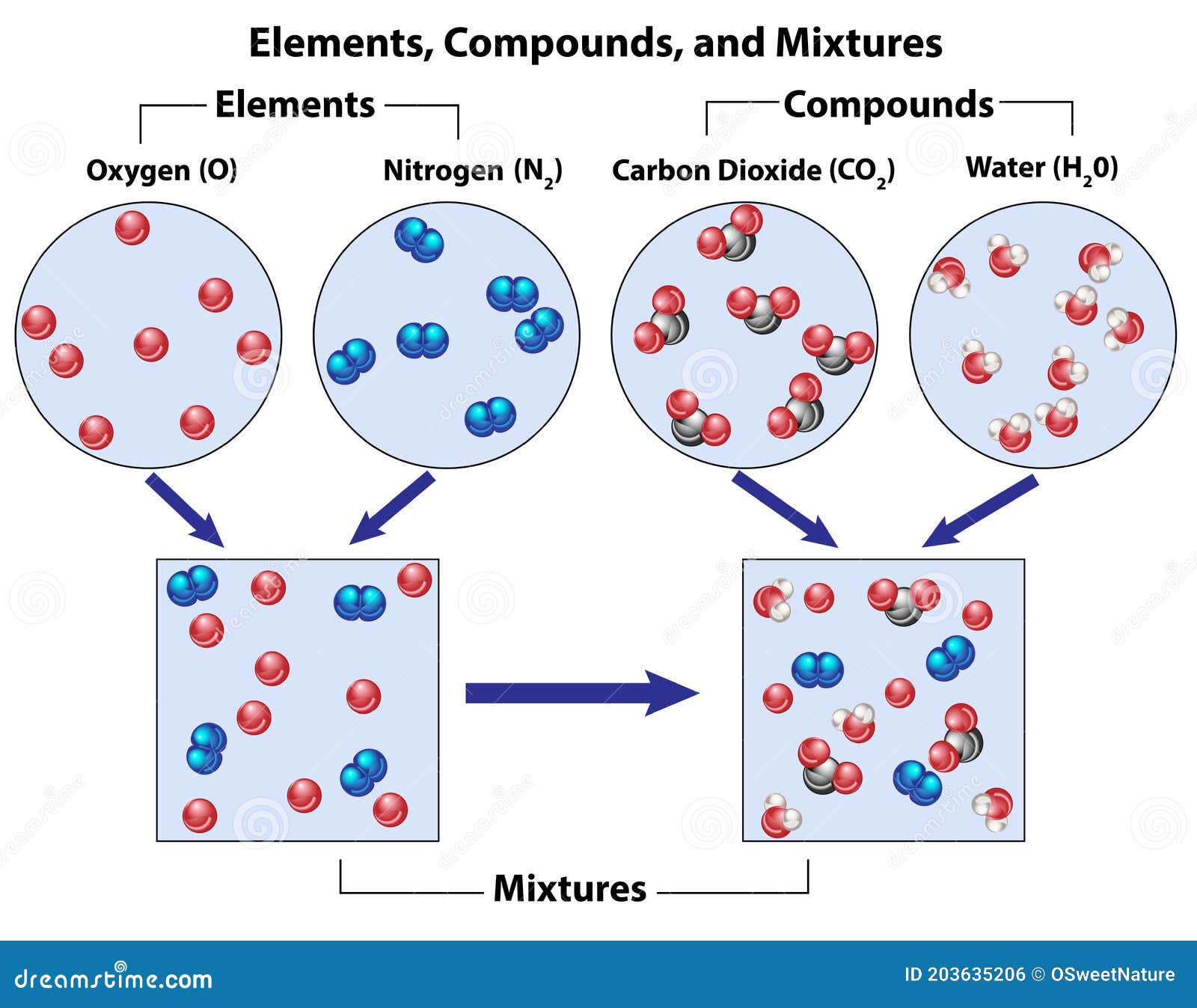
Pure Vinegar Element Compound Or Mixture . It is a mixture that includes substances other than acetic acid and water. If it is pure, the substance is either an element or a compound. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. No, vinegar is not a pure compound. The concentration of the acetic acid is variable. These substances may include trace. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. If a substance is not chemically pure, it is either a. If a substance can be separated into its elements, it is a compound. If a substance can be separated into its elements, it is a compound. If it is pure, the substance is either an element or a compound. Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated.
from diagramlibrarypyx.z19.web.core.windows.net
If it is pure, the substance is either an element or a compound. If a substance can be separated into its elements, it is a compound. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? The concentration of the acetic acid is variable. Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. No, vinegar is not a pure compound. If it is pure, the substance is either an element or a compound. These substances may include trace. It is a mixture that includes substances other than acetic acid and water. If a substance can be separated into its elements, it is a compound.
Which Particle Diagram Represents A Mixture
Pure Vinegar Element Compound Or Mixture Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. If it is pure, the substance is either an element or a compound. The concentration of the acetic acid is variable. These substances may include trace. If a substance can be separated into its elements, it is a compound. If a substance can be separated into its elements, it is a compound. Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? It is a mixture that includes substances other than acetic acid and water. Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. If it is pure, the substance is either an element or a compound. If a substance is not chemically pure, it is either a. No, vinegar is not a pure compound.
From gioodqalc.blob.core.windows.net
Is Vinegar Element Compound Or Mixture at Bryan Peterkin blog Pure Vinegar Element Compound Or Mixture Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. No, vinegar is not a pure compound. If it is pure, the substance is either an element or a compound. If a substance. Pure Vinegar Element Compound Or Mixture.
From cabinet.matttroy.net
Is Pure Table Salt An Element Compound Or Mixture Matttroy Pure Vinegar Element Compound Or Mixture If a substance can be separated into its elements, it is a compound. Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. The concentration of the acetic acid is variable. These substances may include trace. No, vinegar is not a pure compound. If a substance is not chemically. Pure Vinegar Element Compound Or Mixture.
From www.chegg.com
Solved Classify each type of matter as an element, a Pure Vinegar Element Compound Or Mixture The concentration of the acetic acid is variable. If a substance can be separated into its elements, it is a compound. If it is pure, the substance is either an element or a compound. Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. If a substance can be separated into its elements, it is a compound.. Pure Vinegar Element Compound Or Mixture.
From slidetodoc.com
Pure Substances and Mixtures Categorizing Matter All matter Pure Vinegar Element Compound Or Mixture If a substance is not chemically pure, it is either a. If a substance can be separated into its elements, it is a compound. These substances may include trace. If a substance can be separated into its elements, it is a compound. It is a mixture that includes substances other than acetic acid and water. Vinegar is a commonly used. Pure Vinegar Element Compound Or Mixture.
From lunanewsrosales.blogspot.com
Is Vinegar a Compound Element or Mixture Pure Vinegar Element Compound Or Mixture Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. If a substance can be separated into its elements, it is a compound. If it is pure, the substance is either an element or a compound. If it is pure, the substance is either an element or a compound. The concentration of the acetic acid is variable.. Pure Vinegar Element Compound Or Mixture.
From stock.adobe.com
illustration of chemistry, Pure Substances and Mixtures, element Pure Vinegar Element Compound Or Mixture Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. If it is pure, the substance is either an element or a compound. If a substance is not chemically pure, it is either a. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a. Pure Vinegar Element Compound Or Mixture.
From www.studocu.com
Elements, compounds, mixture (answer key) SNC1P Elements, Compounds Pure Vinegar Element Compound Or Mixture Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. It is a mixture that includes substances other than acetic acid and water. If it. Pure Vinegar Element Compound Or Mixture.
From www.youtube.com
Chemical Formula for Vinegar (Acetic Acid or Ethanoic Acid) YouTube Pure Vinegar Element Compound Or Mixture If a substance is not chemically pure, it is either a. If it is pure, the substance is either an element or a compound. If a substance can be separated into its elements, it is a compound. These substances may include trace. If it is pure, the substance is either an element or a compound. Vinegar is a commonly used. Pure Vinegar Element Compound Or Mixture.
From www.pinterest.com
Elements Compounds or Mixtures worksheet Elements compounds and Pure Vinegar Element Compound Or Mixture These substances may include trace. If a substance can be separated into its elements, it is a compound. If it is pure, the substance is either an element or a compound. Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. If it is pure, the substance is either. Pure Vinegar Element Compound Or Mixture.
From circuitdiagramheure.z21.web.core.windows.net
Mixture Of An Element And A Compound Diagram Pure Vinegar Element Compound Or Mixture It is a mixture that includes substances other than acetic acid and water. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. No, vinegar is not a pure compound. If a substance is not chemically pure, it is either a. The concentration of the acetic acid is variable. These substances. Pure Vinegar Element Compound Or Mixture.
From www.numerade.com
SOLVEDWhich of the following figures represents (a) a pure element, (b Pure Vinegar Element Compound Or Mixture If it is pure, the substance is either an element or a compound. Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. These substances may include trace. It is a mixture that includes substances other than acetic acid and water. If a substance can be separated into its. Pure Vinegar Element Compound Or Mixture.
From gioodqalc.blob.core.windows.net
Is Vinegar Element Compound Or Mixture at Bryan Peterkin blog Pure Vinegar Element Compound Or Mixture If a substance is not chemically pure, it is either a. The concentration of the acetic acid is variable. If a substance can be separated into its elements, it is a compound. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? If it is pure,. Pure Vinegar Element Compound Or Mixture.
From www.stfuandplay.com
What do you need to know about heterogeneous and homogeneous mixtures? Pure Vinegar Element Compound Or Mixture If it is pure, the substance is either an element or a compound. Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. If a substance is not chemically pure, it is either a. Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. Vinegar consists. Pure Vinegar Element Compound Or Mixture.
From lunanewsrosales.blogspot.com
Is Vinegar a Compound Element or Mixture Pure Vinegar Element Compound Or Mixture It is a mixture that includes substances other than acetic acid and water. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? If a substance is not chemically pure, it is either a. If a substance can be separated into its elements, it is a. Pure Vinegar Element Compound Or Mixture.
From dearlearners.com
Is Vinegar An Element, Compound, or Mixture? [ANSWERED] Dear Learners Pure Vinegar Element Compound Or Mixture If a substance can be separated into its elements, it is a compound. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? These substances may include trace. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include. Pure Vinegar Element Compound Or Mixture.
From www.slideserve.com
PPT Elements & Compounds PowerPoint Presentation, free download ID Pure Vinegar Element Compound Or Mixture Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? If it is pure, the substance is either an element or a compound. If it. Pure Vinegar Element Compound Or Mixture.
From diagramlibrarypyx.z19.web.core.windows.net
Which Particle Diagram Represents A Mixture Pure Vinegar Element Compound Or Mixture It is a mixture that includes substances other than acetic acid and water. If it is pure, the substance is either an element or a compound. The concentration of the acetic acid is variable. If a substance is not chemically pure, it is either a. Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. Vinegar is. Pure Vinegar Element Compound Or Mixture.
From brainly.com
Is it always possible to identify something as an element, compound Pure Vinegar Element Compound Or Mixture If a substance is not chemically pure, it is either a. No, vinegar is not a pure compound. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? If a substance can be separated into its elements, it is a compound. Vinegar consists of acetic acid. Pure Vinegar Element Compound Or Mixture.
From www.coursehero.com
[Solved] Classify each image as showing a pure element, a pure compound Pure Vinegar Element Compound Or Mixture If it is pure, the substance is either an element or a compound. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? The concentration of the. Pure Vinegar Element Compound Or Mixture.
From sciencenotes.org
Element Non Examples Pure Vinegar Element Compound Or Mixture The concentration of the acetic acid is variable. These substances may include trace. No, vinegar is not a pure compound. If a substance is not chemically pure, it is either a. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. Vinegar is not considered a compound, as it is a. Pure Vinegar Element Compound Or Mixture.
From lunanewsrosales.blogspot.com
Is Vinegar a Compound Element or Mixture Pure Vinegar Element Compound Or Mixture The concentration of the acetic acid is variable. If a substance is not chemically pure, it is either a. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may. Pure Vinegar Element Compound Or Mixture.
From unacademy.com
Science Class 9 Pure Substance vs Mixture UPSC Note on Science Class Pure Vinegar Element Compound Or Mixture It is a mixture that includes substances other than acetic acid and water. If a substance is not chemically pure, it is either a. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? If a substance can be separated into its elements, it is a. Pure Vinegar Element Compound Or Mixture.
From www.thoughtco.com
What Are Examples of Pure Substances? Pure Vinegar Element Compound Or Mixture If a substance is not chemically pure, it is either a. The concentration of the acetic acid is variable. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? Vinegar is not considered a compound, as it is a mixture of acetic acid and water that. Pure Vinegar Element Compound Or Mixture.
From gioodqalc.blob.core.windows.net
Is Vinegar Element Compound Or Mixture at Bryan Peterkin blog Pure Vinegar Element Compound Or Mixture No, vinegar is not a pure compound. If a substance is not chemically pure, it is either a. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? It is a mixture that includes substances other than acetic acid and water. The concentration of the acetic. Pure Vinegar Element Compound Or Mixture.
From chemistryclinic.co.uk
1.8 Elements, compounds, mixtures Chemistry Pure Vinegar Element Compound Or Mixture If a substance can be separated into its elements, it is a compound. If it is pure, the substance is either an element or a compound. If it is pure, the substance is either an element or a compound. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance. Pure Vinegar Element Compound Or Mixture.
From www.compoundchem.com
Compound Interest The sour science of vinegar varieties Pure Vinegar Element Compound Or Mixture Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. The concentration of the acetic acid is variable. If it is pure, the substance is either an element or a compound. No, vinegar is not a pure compound. These substances may include trace. It is a mixture that includes. Pure Vinegar Element Compound Or Mixture.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Pure Vinegar Element Compound Or Mixture Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The concentration of the acetic acid is variable. Vinegar is a commonly used ingredient in cooking and cleaning, but have. Pure Vinegar Element Compound Or Mixture.
From cabinet.matttroy.net
Is Table Salt Sodium Chloride An Element Compound Or Mixture Pure Vinegar Element Compound Or Mixture If a substance is not chemically pure, it is either a. If a substance can be separated into its elements, it is a compound. The concentration of the acetic acid is variable. If it is pure, the substance is either an element or a compound. If it is pure, the substance is either an element or a compound. If a. Pure Vinegar Element Compound Or Mixture.
From gioodqalc.blob.core.windows.net
Is Vinegar Element Compound Or Mixture at Bryan Peterkin blog Pure Vinegar Element Compound Or Mixture No, vinegar is not a pure compound. If a substance can be separated into its elements, it is a compound. Pure substances (element or compound) do not use the terms heterogeneous or homogeneous. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? If it is. Pure Vinegar Element Compound Or Mixture.
From cabinet.matttroy.net
Is Pure Table Salt An Element Compound Or Mixture Matttroy Pure Vinegar Element Compound Or Mixture Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? These substances may include trace. If it is pure, the substance is either an element or a. Pure Vinegar Element Compound Or Mixture.
From www.vrogue.co
Picture Of An Element Compound And Mixture Bmp Clown vrogue.co Pure Vinegar Element Compound Or Mixture If a substance can be separated into its elements, it is a compound. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. If it is pure, the substance is either an element or a compound. Vinegar is not considered a compound, as it is a mixture of acetic acid and. Pure Vinegar Element Compound Or Mixture.
From brainly.in
images of elements compounds and mixtures differences Brainly.in Pure Vinegar Element Compound Or Mixture Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. If a substance can be separated into its elements, it is a compound. It is a mixture that includes substances other than acetic acid and water. If a substance can be separated into its elements, it is a compound.. Pure Vinegar Element Compound Or Mixture.
From temperaturemaster.com
What Category Does Dry Ice? Compound, Element, or Mixture Pure Vinegar Element Compound Or Mixture Vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. The concentration of the acetic acid is variable. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? Pure substances (element or compound) do not. Pure Vinegar Element Compound Or Mixture.
From cabinet.matttroy.net
Is Pure Table Salt An Element Compound Or Mixture Matttroy Pure Vinegar Element Compound Or Mixture If a substance can be separated into its elements, it is a compound. If it is pure, the substance is either an element or a compound. No, vinegar is not a pure compound. Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? These substances may. Pure Vinegar Element Compound Or Mixture.
From slideplayer.com
Elements, Compounds & Mixtures! Mr. Coffey. ppt download Pure Vinegar Element Compound Or Mixture Vinegar is a commonly used ingredient in cooking and cleaning, but have you ever wondered whether it is a pure substance or a mixture? If a substance can be separated into its elements, it is a compound. It is a mixture that includes substances other than acetic acid and water. The concentration of the acetic acid is variable. Vinegar is. Pure Vinegar Element Compound Or Mixture.