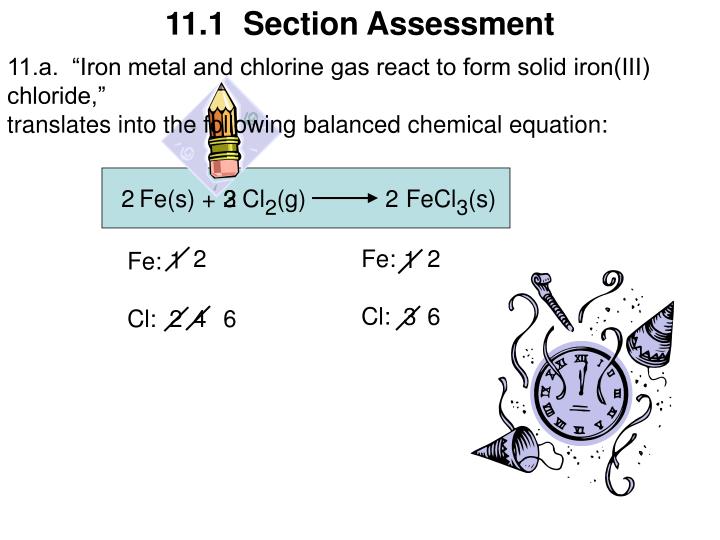
Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation . 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2 fe + 3 cl2 → 2 fecl3. 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in the equation: 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: (i) calculate the maximum mass of iron (iii). Here down below is the equation. (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of.
from www.slideserve.com
Iron (iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3. (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Here down below is the equation. (i) calculate the maximum mass of iron (iii). Iron (iii) chloride can be produced by the reaction shown in this equation:
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952
Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Here down below is the equation. 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2 fe + 3 cl2 → 2 fecl3. 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. (i) calculate the maximum mass of iron (iii). Iron (iii) chloride can be produced by the reaction shown in this equation: Here down below is the equation. (b) iron(iii) chloride can be produced by the reaction shown in the equation:
From www.numerade.com
SOLVED If 23 grams of iron (III) chloride reacts with 41 grams of Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in this equation: (i) calculate the maximum. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation (i) calculate the maximum mass of iron (iii). 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in this equation: (b) iron(iii) chloride can be produced by the reaction shown in the. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.numerade.com
Iron(III) chloride can be prepared by reacting iron metal with chlorine Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation (i) calculate the maximum mass of iron (iii). 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3. Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in this equation: (b) iron(iii) chloride can. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.numerade.com
SOLVED In water, iron(III) chloride reacts with sodium hydroxide Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3. 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3. 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: (i) calculate the maximum mass of iron (iii). (b) iron(iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Here down below is the equation. 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation:. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.youtube.com
Iron III Chloride Reaction With Potassium Thiocyanate (FeCl3 + KSCN Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Iron (iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: (i) calculate the maximum mass of iron (iii). Iron (iii) chloride can be produced by the reaction shown in this equation: Here down below is the equation. Iron (iii) chloride can be produced by. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.chegg.com
Solved The reaction of iron(III) chloride with tin(II) Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation (b) iron(iii) chloride can be produced by the reaction shown in the equation: (i) calculate the maximum mass of iron (iii). 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in the equation: 4 (b) iron(iii) chloride can be produced by the reaction shown in. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.chegg.com
Solved When iron (III) chloride and hydrogen sulfide react Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3. Iron (iii) chloride can be produced by the reaction shown in the equation: Here down below is the equation. 2 fe + 3 cl 2 →. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.youtube.com
How to Write the Formula for Iron (III) chloride YouTube Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. Here down below is the equation. 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3. 2 fe +. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. (b) iron(iii) chloride can be produced by the reaction shown in the equation: (b). Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.numerade.com
Write net ionic equations for the reactions depicted in photo (a Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation (b) iron(iii) chloride can be produced by the reaction shown in the equation: Here down below is the equation. 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3. Iron (iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl 2. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952 Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation (i) calculate the maximum mass of iron (iii). Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. (b) iron(iii) chloride can be produced by the reaction shown in the equation: 4 (b) iron(iii) chloride can be produced by the reaction shown in. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.youtube.com
Chemistry 110, Experiment 15 Part Two Oxidation of Alcohols, Iron Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in this equation: Here down below is. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From slideplayer.com
“Chemical Reactions”. ppt download Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. (i) calculate the maximum mass of iron (iii). Here down below is the equation. 2 fe. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.youtube.com
Equation for FeCl3 + H2O Iron (III) chloride + Water YouTube Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl2 → 2 fecl3. Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in this equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in the equation:. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.numerade.com
SOLVEDWhat would be formed by the electrolysis of an acidic iron(III Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Here down below is the equation. 2 fe + 3 cl 2 → 2 fecl 3. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.youtube.com
How to write the formula for iron (III) chloride YouTube Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl2 → 2 fecl3. Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in the equation: Here down below is the equation. 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. (b) iron(iii) chloride can be produced. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.bartleby.com
The reaction of iron metal and chlorine gas to give iron(III) chloride Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Iron (iii) chloride can be produced by the reaction shown in this equation: Here down below is the equation. 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3. (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 →. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.youtube.com
Lewis Structure of Iron (III) Chloride, FeCl3 YouTube Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: (i) calculate the maximum mass of iron (iii). Iron (iii) chloride can be produced by the reaction shown in this equation: Here down below is the equation. Iron (iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl 2. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.slideserve.com
PPT Oral assessment PowerPoint Presentation, free download ID3444733 Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in this equation: 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.numerade.com
SOLVEDThe reaction of iron metal and chlorine gas to give iron(III Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Here down below is the equation. Iron (iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in this equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.coursehero.com
[Solved] When iron (III) chloride and hydrogen sulfide react in the gas Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2 fe + 3. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.coursehero.com
[Solved] Iron will react with chlorine to form iron(III) chloride as Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl2 → 2 fecl3. (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. 4 (b) iron(iii) chloride can be produced by the reaction shown in. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From slideplayer.com
Chemical Reactions. ppt download Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in this equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: (i) calculate the maximum mass of iron (iii). 2 fe + 3 cl2 → 2 fecl3. Here down below is the. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From pandai.me
Chemical Formulae Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. (b) iron(iii) chloride can be produced by the reaction shown in the equation: Here down below is the equation. (i) calculate the maximum mass of iron (iii). Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.bartleby.com
Answered The Iron metal reacts with chlorine gas… bartleby Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. (i) calculate the maximum mass of iron (iii). 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.numerade.com
SOLVED (10 points) In an acidic solution; Iron (II) chloride reacts Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Here down below is the equation. 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. (i) calculate the maximum mass of iron (iii). Iron (iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.chegg.com
Solved Reaction 10 Aqueous iron(III) chloride + aqueous Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 4 (b) iron(iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. 2 fe + 3 cl2 → 2 fecl3. Iron (iii) chloride can be produced by the reaction shown in. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.slideserve.com
PPT Double Replacement Reactions PowerPoint Presentation, free Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Iron (iii) chloride can be produced by the reaction shown in this equation: Iron (iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in this equation: (i) calculate the maximum mass. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From slideplayer.com
Chapter 4 Chemical Reactions ppt download Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. (b) iron(iii) chloride can be produced by the reaction shown in the equation: Iron (iii) chloride can be produced by the reaction shown in this equation: 4 (b) iron(iii) chloride can be produced. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From testbook.com
Iron (III) Chloride Formula Know Structure, Preparation,& Uses Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. (b) iron(iii) chloride can be produced by the reaction shown in the equation: (b) iron(iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.numerade.com
SOLVEDIron(III) chloride is produced by the reaction of iron and Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in the equation: 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2 fe + 3 cl2 → 2 fecl3 (i) calculate the maximum mass of. Here. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation, free Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation 2 fe + 3 cl2 → 2 fecl3. 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. Iron (iii) chloride can be produced by the reaction shown in the equation: Here down below is the equation. 2 fe + 3 cl 2 → 2 fecl 3 (i) calculate the maximum mass of. 2. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.
From www.youtube.com
Iron(iii) Chloride Preparation YouTube Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation Here down below is the equation. Iron (iii) chloride can be produced by the reaction shown in this equation: 2 fe + 3 cl2 → 2 fecl3. (b) iron(iii) chloride can be produced by the reaction shown in the equation: (i) calculate the maximum mass of iron (iii). Iron (iii) chloride can be produced by the reaction shown in the. Iron(Iii) Chloride Can Be Produced By The Reaction Shown In The Equation.